5308
Impact of Physiological Noise on Serial Correlations in Fast Simultaneous Multislice (SMS) EPI at 7T1Centre for Advanced Imaging, The University of Queensland, Brisbane, Australia, 2Queensland Brain Institute, The University of Queensland, Brisbane, Australia
Synopsis
We investigated the influence of physiological noise on statistical inference in fMRI at the single-subject level. By comparing two SMS sequences with a short and a long TR, we explored the interaction between repetition time, physiological noise modelling and the autoregressive model used to characterize serial correlations in fMRI data. Using variational Bayesian inference, we found that fMRI acquisitions with a short TR require accurate modelling of cardiac and respiratory processes to successfully remove serial correlations from the fMRI time series. For the SMS sequence with a longer TR, the standard AR model of order 1 proved sufficient.
Purpose
We investigate the influence of physiological noise on statistical inference in fMRI at the single-subject level. Specifically, we explore the interaction between repetition time (TR), physiological noise modelling and the autoregressive (AR) model used to characterize serial correlations in fMRI data. Serial correlations in fMRI, i.e. temporal auto-correlations in the error time series, are one of the key issues in drawing inference, because they affect both the model fitting and the statistical analysis1. Underestimating the variance can lead to inflated t-values and, hence, increased false positive rates2. Pre-whitening the fMRI time series using an AR model of order 1 in combination with physiological noise modelling is a successful strategy to improve the validity of drawn inference3. However, with the considerable reduction in TR up to one order of magnitude through the development of fast SMS acquisitions4,5, higher AR model orders are required even when including RETROICOR6 regressors7. Furthermore, we investigate whether additional physiological noise regressors based on the respiration (RRF)8 and cardiac response function (CRF)9 can reduce the optimal AR model and hence, remove serial correlations from SMS data. Furthermore, we compare these results to an acquisition with a longer, more traditional volume TR.Methods
We acquired MRI data on a MAGNETOM 7T whole-body scanner (Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil (Nova Medical, Wilmington, US) using the CMRR SMS sequence implementation (release 11a)4,5 with a short TR of 589 ms and long TR of 1990 ms, respectively (Table 1). After approval by the local human ethics committee and written informed consent, 3 subjects performed a 6-minute rhythmic finger-tapping task. Preprocessing and statistical analysis were performed in Matlab10 using SPM1211, including realignment, smoothing (5mm) and physiological noise modelling12. Using the Variational Bayesian framework1, we estimated the optimal AR model order ranging from 1 to 10 without physiological noise modelling, when including RETROICOR regressors only, and when including RETROICOR, RRF and CRF regressors. To assess the evidence for a higher order AR model13, we computed the log Bayes factor between an AR model of order 4 to the common AR(1) model.Results
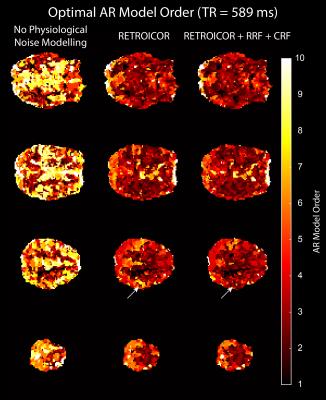
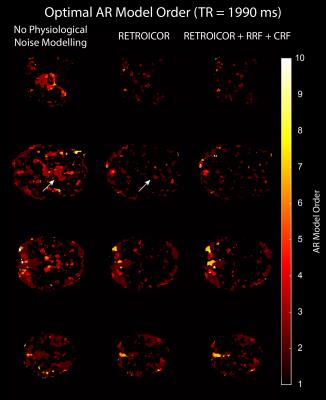
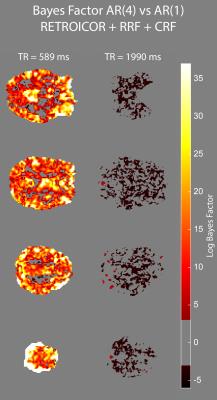
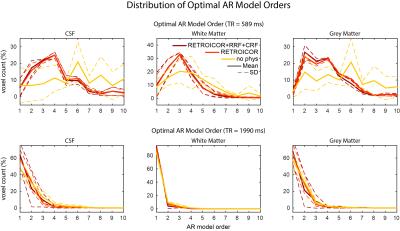
Physiological noise modelling successfully reduced the optimal AR model order for the short-TR sequence. In general, RETROICOR regressors induced the largest reduction from up to order 10 to around 4. The impact of RRF and CRF regressors was more localized (Figure 1). In contrast, the optimal AR model order for the long-TR sequence was low even without physiological noise modelling (Figure 2). RETROICOR regressors slightly reduced the optimal AR model order in subcortical and CSF-bearing areas. For the short-TR sequence, we found strong evidence for an AR model of order 4 even when including physiological noise regressors in CSF and grey matter areas (Figure 3). The long-TR sequence showed mostly support for an AR-model of order 1. Figure 4 illustrates the distribution of optimal AR model orders across subjects in different tissue classes. CSF showed the highest AR model order, white matter the lowest and grey matter voxel range in-between.Discussion
While including physiological noise regressors successfully reduced the optimal AR model order for sequences with short TR, some serial correlations remain. Given that RETROICOR regressors are a Fourier expansion based on the periodic properties of these signals, a noise model also allowing changes in shape and amplitude of cardiac and respiratory signals might better explain remaining correlations14. Furthermore, the performance of respiratory and cardiac response functions can be improved when using a subject specific response15.Conclusion
fMRI acquisitions with short TR require an advanced noise handling when drawing inference on the single-subject level. Particularly, physiological noise from cardiac and respiratory activity needs to be accurately modelled to successfully remove serial correlations from the fMRI time series. While sequences with longer TR certainly profit from sophisticated noise handling, an AR model of order 1 proved sufficient.Acknowledgements
We would like to thank Will Penny for helpful advice on the interpretation of the variational Bayesian inference results.
MB acknowledges funding from ARC Future Fellowship grant FT140100865. The authors acknowledge the facilities of the National Imaging Facility at the Centre for Advanced Imaging, University of Queensland and the scientific support of Siemens Ltd, Bowen Hills, Australia.
References
1 Penny, W., Kiebel, S., Friston, K., 2003. Variational Bayesian inference for fMRI time series. NeuroImage 19, 727–741. doi:10.1016/S1053-8119(03)00071-5
2 Purdon, P.L., Weisskoff, R.M., 1998. Effect of temporal autocorrelation due to physiological noise and stimulus paradigm on voxel-level false-positive rates in fMRI. Hum. Brain Mapp. 6, 239–249.
3 Lund, T.E., Madsen, K.H., Sidaros, K., Luo, W.-L., Nichols, T.E., 2006. Non-white noise in fMRI: Does modelling have an impact? NeuroImage 29, 54–66. doi:10.1016/j.neuroimage.2005.07.005
4 Feinberg, D.A., Moeller, S., Smith, S.M., Auerbach, E., Ramanna, S., Glasser, M.F., Miller, K.L., Ugurbil, K., Yacoub, E., 2010. Multiplexed Echo Planar Imaging for Sub-Second Whole Brain FMRI and Fast Diffusion Imaging. PLoS ONE 5, e15710. doi:10.1371/journal.pone.0015710
5 Moeller, S., Yacoub, E., Olman, C.A., Auerbach, E., Strupp, J., Harel, N., Ugurbil, K., 2010. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 63, 1144–1153. doi:10.1002/mrm.22361
6 G. H. Glover, T. Q. Li, and D. Ress, “Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR,” Magn. Reson. Med., vol. 44, no. 1, pp. 162–167, Jul. 2000.
7 Bollmann, S., Pucket, A., Cunnington, R., Barth, M., 2016. Serial correlations in ultra-fast simultaneous multislice (SMS) EPI at 7T, in: Proceedings of the European Society for Magnetic Resonance in Medicine and Biology. Presented at the ESMRMB, Vienna, Austria, p. 334.
8 Birn, R.M., Smith, M.A., Jones, T.B., Bandettini, P.A., 2008. The respiration response function: The temporal dynamics of fMRI signal fluctuations related to changes in respiration. NeuroImage 40, 644–654. doi:10.1016/j.neuroimage.2007.11.059
9 Chang, C., Cunningham, J.P., Glover, G.H., 2009. Influence of heart rate on the BOLD signal: The cardiac response function. NeuroImage 44, 857–869. doi:10.1016/j.neuroimage.2008.09.029
10 Matlab 2014b, The MathWorks, Inc., Natick, Massachusetts, United States
11 SPM 12, Wellcome Trust Centre for Neuroimaging, London, UK
12 L. Kasper, S. Bollmann, C. Hutton, J. Heinzle, A. O. Diaconescu, K. P. Pruessmann, and K. E. Stephan, “The PhysIO Toolbox for Preprocessing and Noise Modeling of Physiological Data in fMRI,” presented at the Organization for Human Brain Mapping, Honolulu, USA, 2015, p. 3736.
13 Kass, R.E., Raftery, A.E., 1995. Bayes Factors. J. Am. Stat. Assoc. 90, 773–795. doi:10.1080/01621459.1995.10476572
14 Falahpour, M., Refai, H., Bodurka, J., 2013. Subject specific BOLD fMRI respiratory and cardiac response functions obtained from global signal. NeuroImage 72, 252–264. doi:10.1016/j.neuroimage.2013.01.050
15 Särkkä, S., Solin, A., Nummenmaa, A., Vehtari, A., Auranen, T., Vanni, S., Lin, F.-H., 2012. Dynamic retrospective filtering of physiological noise in BOLD fMRI: DRIFTER. NeuroImage 60, 1517–1527. doi:10.1016/j.neuroimage.2012.01.067
Figures