5299
Lower Resting Cerebral Blood Flow but Greater Cerebrovascular Reactivity in Young Adults with Higher Aerobic FitnessCatherine Foster1, Jessica J Steventon1,2, Daniel Helme1, Valentina Tomassini3, and Richard G Wise1
1School of Psychology, Cardiff University Brain Research Imaging Centre, Cardiff, United Kingdom, 2School of Medicine, National Mental Health Research Institute, Cardiff, United Kingdom, 3School of Medicine, Institute of Psychological Medicine and Clinical Neurosciences, Cardiff, United Kingdom
Synopsis
We measured the association between aerobic fitness and cerebral blood flow (CBF) and cerebrovascular reactivity (CVR) in young, healthy adults using multiple inversion time (MTI) arterial spin labelling (ASL), with a hypercapnic challenge to assess CVR. The results show that higher fitness is associated with lower baseline CBF and greater CVR. Although studies with a larger sample size are required to clarify the relationship between fitness and cerebrovascular function in early adulthood, the current results suggest that aerobic fitness may promote vascular efficiency and reserve.
Purpose
Accumulating evidence suggests that aerobic fitness promotes cerebrovascular function and neuroplasticity1, 2, protecting against age-related decline and potentially slowing neurological disease onset3. Still, there is limited understanding of both the biological mechanisms and the extent to which fitness exerts neuroprotective effects. Research efforts to date have been focused on older adults and patient populations; but, it is necessary to study the effects of fitness on cerebrovascular function in young, healthy adults to understand the lifelong effects of physical activity. This cross-sectional study in young adults used arterial spin labelling (ASL) fMRI to examine differences related to aerobic fitness in cerebral blood flow (CBF) and cerebrovascular reactivity (CVR) to CO2, which are measures of overall cerebrovascular health.Methods
20 participants (11 females, mean age = 25.4±4.6), eligible to undergo intensive exercise and respiratory gas modulations, completed a VO2peak test to measure aerobic fitness and underwent 3T fMRI (GE HDx system). CBF data was acquired at baseline and during hypercapnia using multiple inversion time (MTI) pulsed ASL (TIs: 400, 500, 600, 700, 1100, 1400, 1700 and 2000ms, TE = 2.7ms; PICORE QUIPSS II4 cut-off at TI=700 ms, TI2 = 300ms, dual-echo gradient-echo readout5 and spiral k-space acquisition6). A variable TR was used for efficiency (min. 100ms for short TIs, min. 1600ms for long TIs), 15 slices, resolution = 3.1x3.1x8.4mm3, matrix size = 64x64mm, FOV = 19.8cm, flip angle = 20°. For the hypercapnia challenge (target +5 mmHg PETCO2), gas mixtures of 5% CO2 were delivered using the prospective control method, breathing circuit described previously7. Perfusion quantification was performed using a two-compartment model7 for grey matter (GM). CBF CVR was calculated as the unit change in CBF per unit change in PETCO2. Region of interest (ROI) analysis of GM was performed using BASIL within FSL8 and followed up with voxelwise analysis using FSL Randomise9.Results
CBF or CVR were not predicted by gender, age or body mass index (F(3, 16) = 0.216, p > 0.05, R2 = 0.039, R2Adjusted = -.141).Correlation analysis between VO2peak, CBF and CVR (ROI GM) revealed a non-significant inverse correlation between VO2peak and CBF at rest; r = -.4, p = 0.07, p’ = 0.17 and during hypercapnia; r = -.23, p = 0.33, p’ = 0.58. There was a significant positive correlation between VO2peak and CVR; r = .62, p = 0.004, p’ = 0.009. The PETCO2 response was not driven by VO2peak; r = -.26, p = 0.27, nor was CVR by resting CBF; r = -.14, p = 0.55 (Figure 1). Follow-up voxelwise analysis of GM to further explore the ROI trends showed a significant inverse association between VO2peak and CBF at rest in portions of the thalamus, brainstem, visual cortex, precuneous and cerebellum; this relationship was not present for CBF during hypercapnia (Figure 2) where a significant inverse association was found in the frontal pole. Voxelwise GM CVR was not strongly correlated with VO2peak.Conclusions
This novel demonstration of an inverse relationship between GM CBF and VO2peak and positive association between GM CVR and VO2peak in young, healthy adults suggests that aerobic fitness may benefit brain health early in life. While the CBF results may seem counterintuitive as higher fitness is known to increase CBF in older adults, it is possible that exercise counteracts the overall CBF decline seen in ageing but reflects a cerebrovascular efficiency mechanism in early adulthood. It is known that physical training increases the ability to extract oxygen from tissue peripherally, a similar central mechanism would result in lower CBF requirements. A second possibility is that high aerobic fitness may increase capillary density through angiogenesis which would translate to a smaller diffusion distance and lower CBF. CVR is higher with increased fitness but baseline CBF is lower; this may be driven by a greater vasodilatory or vascular reserve capacity resulting from fitness induced vascular plasticity. The PETCO2 increase during hypercapnia was not strongly related to VO2peak although the % hypercapnic CBF change was, this suggests subjects with higher fitness were more sensitive to any change in PETCO2 which again supports the idea of fitness induced vascular plasticity. The VO2peak correlations with CBF and CVR require replication in a larger sample to determine a true significant effect. Nevertheless, these results suggest maintenance of physical fitness may be important for optimal cerebrovascular function at all ages.Acknowledgements
This work was supported by the Wellcome Trust and Cardiff University.References
1. Voss, M. W. et al. (2013). Bridging animal and human models of exercise-induced brain plasticity. Trends in cognitive sciences, 17(10), 525-544. 2. Gauthier, C. J. et al. (2015). Hearts and minds: linking vascular rigidity and aerobic fitness with cognitive aging. Neurobiology of aging, 36(1), 304-314. 3. Ahlskog, J. E., Geda, Y. E., Graff-Radford, N. R., & Petersen, R. C. (2011, September). Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. In Mayo Clinic Proceedings, Vol. 86(9), pp. 876-884. 4. Wong, E. C., Buxton, R. B., & Frank, L. R. (1998). Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magnetic resonance in medicine, 39(5), 702-708. 5. Liu, T. T., Wong, E. C., Frank, L. R., & Buxton, R. B. (2002). Analysis and design of perfusion-based event-related fMRI experiments. NeuroImage,16(1), 269-282. 6. Glover, G. H. (1999). Simple analytic spiral K-space algorithm. Magnetic Resonance in medicine, 42(2), 412-415. 7. Tancredi, F. B., Lajoie, I., & Hoge, R. D. (2014). A simple breathing circuit allowing precise control of inspiratory gases for experimental respiratory manipulations. BMC research notes, 7(1), 1. 8. Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M., & Nichols, T. E. (2014). Permutation inference for the general linear model. Neuroimage, 92, 381-397. 9. Woolrich, M. W., Jbabdi, S., Patenaude, B., Chappell, M., Makni, S., Behrens, T. ... & Smith, S. M. (2009). Bayesian analysis of neuroimaging data in FSL. Neuroimage, 45(1), S173-S18.Figures
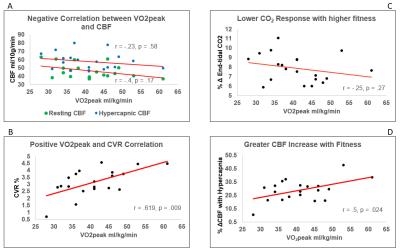
Figure
1. There was an inverse trend towards reduced
resting CBF in subjects with higher fitness, the same trend was observed during
hypercapnia (A). CVR to CO2 was significantly higher in subjects with higher
fitness (B). This effect was not driven by an effect of fitness on the CO2
response to CO2 (C) but by the greater CBF response to an increase
in PETCO2 in subjects with higher
aerobic fitness (D).
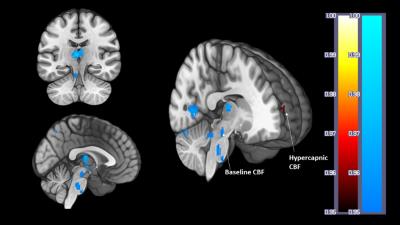
Figure 2. Results from the
voxelwise analysis showing clusters where CBF was inversely correlated with fitness
at baseline in the thalamus, brainstem, precuneous, visual cortex, lingual
gyrus, and during hypercapnia in the frontal pole.