5297
Morphological And Functional Research In Parkinson Disease By Magnetic Resonance Imaging1Department of Radiology, the First Hospital of Lanzhou University, Lanzhou, People's Republic of China
Synopsis
Parkinson's disease (PD) is the second most common neurodegenerative disease after AD, and the most frequent subcortical degenerative disease.We hope to found morphological and functional characteristic change in PD
abstract
Purpose: Parkinson disease(PD) is accompanied by degeneration of the nigrostriatal dopaminergic system,with neuronal loss and reactive gliosis in the substantia nigra found at autopsy. Whereas PD are characterized by the general neuronal loss of the dopaminergic system,a high percentage of surviving neurons contain intracellular inclusions in the form of Lewy body,similar to other neurodegenerative disorders1-3.
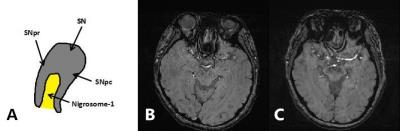
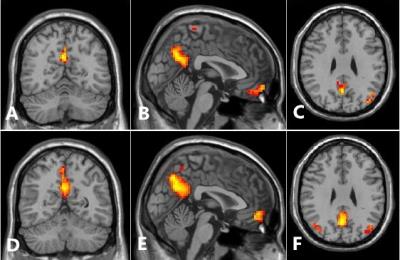
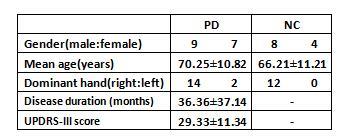
Method: PD and normal control group(NC) study to evaluate nigrosome-1 from substantia nigra ,underwent susceptibility weighted imaging(SWI) at 3T MRI scanner (MAGNETOM Skyra, Siemens Healthcare)in 16 PD patients and 12 NC were scanned(Sequence:3D GRE-SWI,TR:27ms,TE:20ms,Flip angle:15deg,Matrix size:256×256).The bilateral substantia nigra was evaluated by two neuroradiologists for the presence,absence or indecisive presence of nigrosome-14-13. In the meantime,we using blood oxygen level dependent(BOLD) (Slice:40,Slice thickness:3mm,TR:3200ms,TE:30ms,Flip angle:90deg,Matrix size:64×64)to investigate differences in high function area within the DMN14-16 in patients with PD and NC by regional homogeneity,we base on cluster size and morphometry to test differences in these two group by functional MRI,try to discover an result to answer what kind of relationship between structural abnormalities and function disoder17,18.
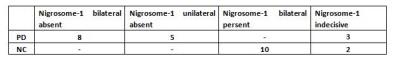
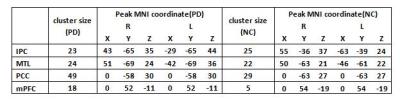
Results: We identified nigrosome-1 in NC and it was not identified in the PD,the swallow-tail sign was discriminated.Meanwhile,our findings persent a functional disorder of the DMN in PD patients,there were not significant change in inferior parietal cortex(IPC) and middle temporal lobe(MTL) by the two groups,but posterior cingulate cortex(PCC) and medial prefrontal cortex(mPFC) were obviously increased(p<0.05) by one-sample t-test and gaussian random field(GRF) correction(voxel-level p and cluster-level p<0.05).
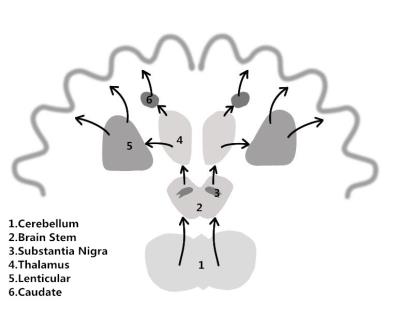
Discussion: Parkinson's disease is based on the degeneration of the dopaminergic neurons in substantia nigra, because of reduce the secretion of inhibitory neurotransmitter,the balance of excitement and inhibition in substantia nigra-striatum pathways is upset, extrapyramidal reactions is caused by hyperexcitability and transmit to the motor cortex by nerve pathways and the corresponding clinical symptoms19-26. Conclusion: In the absence of significant differences between PD and NC group.Interosculation morphology and function research maybe could explain the clinic term a disorder with prominent bradykinesia and mutple extrapyramidal symptoms.
Acknowledgements
The authors thank Siemens Healthcare for technical assistanceReferences
[1] Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease[J]. Mov Disord, 2015,30(12):1591-1601. [2] Dickson DW. Parkinson's disease and parkinsonism: neuropathology[J]. Cold Spring Harb Perspect Med, 2012,2(8). [3] Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity[J]. Brain, 1991,114 ( Pt 5):2283-2301. [4] Young IR, Bailes DR, Burl M, et al. Initial clinical evaluation of a whole body nuclear magnetic resonance (NMR) tomograph[J]. J Comput Assist Tomogr, 1982,6(1):1-18. [5] Reichenbach JR, Schweser F, Serres B, et al. Quantitative Susceptibility Mapping: Concepts and Applications[J]. Clin Neuroradiol, 2015,25 Suppl 2:225-230. [6] Tocchio S, Kline-Fath B, Kanal E, et al. MRI evaluation and safety in the developing brain[J]. Semin Perinatol, 2015,39(2):73-104. [7] Wang Y, Butros SR, Shuai X, et al. Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging[J]. AJNR Am J Neuroradiol, 2012,33(2):266-273. [8] von LF, Werner C, Jörn T, et al. T2*-weighted MRI in diagnosis of multiple system atrophy. A practical approach for clinicians[J]. J Neurol, 2007,254(9):1184-1188. [9] Haacke EM, Mittal S, Wu Z, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1[J]. AJNR Am J Neuroradiol, 2009,30(1):19-30. [10] Haacke EM, Xu Y, Cheng YC, et al. Susceptibility weighted imaging (SWI)[J]. Magn Reson Med, 2004,52(3):612-618. [11] Schneider E, Ng KM, Yeoh CS, et al. Susceptibility-weighted MRI of extrapyramidal brain structures in Parkinsonian disorders[J]. Medicine (Baltimore), 2016,95(26):e3730. [12] Meijer FJ, Steens SC, van Rumund A, et al. Nigrosome-1 on Susceptibility Weighted Imaging to Differentiate Parkinson's Disease From Atypical Parkinsonism: An In Vivo and Ex Vivo Pilot Study[J]. Pol J Radiol, 2016,81:363-369. [13] Schwarz ST, Afzal M, Morgan PS, et al. The 'swallow tail' appearance of the healthy nigrosome - a new accurate test of Parkinson's disease: a case-control and retrospective cross-sectional MRI study at 3T[J]. PLoS One, 2014,9(4):e93814. [14] Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease[J]. Ann N Y Acad Sci, 2008,1124:1-38. [15] Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis[J]. Proc Natl Acad Sci U S A, 2003,100(1):253-258. [16] Mohan A, Roberto AJ, Mohan A, et al. The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review[J]. Yale J Biol Med, 2016,89(1):49-57. [17] Skidmore FM, Yang M, Baxter L, et al. Apathy, depression, and motor symptoms have distinct and separable resting activity patterns in idiopathic Parkinson disease[J]. Neuroimage, 2013,81:484-495. [18] Skidmore FM, Yang M, Baxter L, et al. Reliability analysis of the resting state can sensitively and specifically identify the presence of Parkinson disease[J]. Neuroimage, 2013,75:249-261. [19] Lim E. A walk through the management of Parkinson s disease[J]. Ann Acad Med Singapore, 2005,34(2):188-195. [20] Lieu CA, Subramanian T. The interhemispheric connections of the striatum: Implications for Parkinson's disease and drug-induced dyskinesias[J]. Brain Res Bull, 2012,87(1):1-9. [21] Utter AA, Basso MA. The basal ganglia: an overview of circuits and function[J]. Neurosci Biobehav Rev, 2008,32(3):333-342. [22] Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function[J]. Annu Rev Neurosci, 2009,32:413-434. [23] Warnaar P, Couto J, Negrello M, et al. Duration of Purkinje cell complex spikes increases with their firing frequency[J]. Front Cell Neurosci, 2015,9:122. [24] Percheron G, François C, Talbi B, et al. The primate motor thalamus[J]. Brain Res Brain Res Rev, 1996,22(2):93-181. [25] Hoshi E, Tremblay L, Féger J, et al. The cerebellum communicates with the basal ganglia[J]. Nat Neurosci, 2005,8(11):1491-1493. [26] Sokolov AA, Erb M, Gharabaghi A, et al. Biological motion processing: the left cerebellum communicates with the right superior temporal sulcus[J]. Neuroimage, 2012,59(3):2824-2830.Figures