5296
An fMRI-based neurologic signature of lower back pain1Peking University First Hospital, Beijing, People's Republic of China, 2Philips Healthcare, Beijing China, 3Philips Research China, Shanghai, China, 4Philips Healthcare, Hongkong China
Synopsis
Brain function MRI (fMRI) could successfully demonstrate that differences in the pattern of brain activity to lower back pain (LBP) can be used as a neurological marker to distinguish between individuals with and without LBP. Medical, legal and business professionals have recognized the importance of this research topic and of developing objective measures of LBP and other chronic pain.
Purpose
Lower back pain (LBP) due to hernia of lumbar disc is one common type of chronic pain. However, the neurological markers, critical to diagnosis of LBP, are still largely unknown. The purpose of this study is to explore the ability of multivariate pattern analysis of resting state-functional MRI (rs-fMRI) to objectively identify individuals with LBP from healthy subjects.Material and Methods
MRI examinations were performed on 20 LBP patients and 18 age and gender-matched normal controls (NC). Both 3D-T1WI and rs-fMRI were acquired by using a 3.0T MR scanner (Philips Achieva TX, Best, The Netherlands). Pain degrees of all the patients were assessed by a mechanical numerical rating scale (NRS). The amplitude of low-frequency fluctuations (ALFF), fractional ALFF (fALFF), regional homogeneity (ReHo), and several network parameters (clustering coefficient (Cp), characteristic path length (Lp), local efficiency (Eloc), global efficiency (Eg), network synchronization, and node degree) were calculated and extracted as classification features for each subject. Then, multiple classifiers (including naive bayes, random forest, artificial neural network and support vector machine) were evaluated and compared.Results
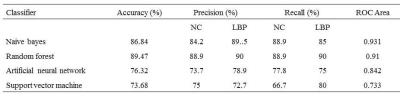
Fisher score was performed for the feature reduction on ALFF, fALFF, ReHo and network parameters. Finally, the selected ALFF, fALFF, Reho values within different brain regions (as shown in Table 1) and network parameters (Eloc, Cp, network synchronization and node degree) were selected as the final features for classification. The classification results were summarized in Table 2. Both Naïve bayes and random forest showed a good classification performance with accuracies of 86.84% and 89.47% respectively. And the random forest showed the best performance with the accuracy of 89.47%, precision of 90% and 88.9% for LBP and NC, and recall of 90% and 88.9% for LBP and NC respectively.Discussion
Classifiers such as naive bayes, random forest, artificial neural network and support vector machine have been proved to be effective in the classification and prediction of clinical diseases such. And fMRI has been widely used in the study of neuro-related diseases. Features extracted from fMRI datasets are very important to character the brain function and furtherly can be used to predict the stage and development of the disease. In this study, the results showed the ability of the classifier for the differentiation of LBP patients from normal controls, which would be helpful for the diagnosis of the disease by using fMRI in addition to the clinical evaluation. The random forest showed the best performance compared to other classifiers, which indicated that this kind of classifier is proper for the differentiation of LBP patient by using the selected features.Conclusion
This technique demonstrates that differences in the pattern of brain activity to LBP can be used as a neurological marker to distinguish between individuals with and without LBP. Medical, legal and business professionals have recognized the importance of this research topic and of developing objective measures of LBP and other chronic pain. This method of data analysis was very successful in correctly classifying each of the two groups.Acknowledgements
No acknowledgement found.References
[1] Achard S, Bullmore E. (2007). Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3, e17.
[2] Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. (2005). Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9, 463–484.
[3] Cauda F, Sacco K, Duca S, Cocito D, D'Agata F, Geminiani GC, Canavero S. (2009). Altered resting state in diabetic neuropathic pain. PLoS One 4, e4542.
[4] Farmer MA, Baliki MN, Apkarian AV. (2012). A dynamic network perspective of chronic pain. Neurosci Lett 520,197–203.
Figures