5294
Subthalamic nucleus activation under audio-motor transformation in lateralized Parkinson’s disease1Human and Animal Physiology, Taras Shevchenko National University of Kyiv, Kyiv, Ukraine, 2Medical Clinic BORIS, Kyiv, Ukraine, 3Department of extrapyramidal disorders, D. F. Chebotarev Institute of Herontology, Kyiv, Ukraine, 4Taras Shevchenko National University of Kyiv, Kyiv, Ukraine
Synopsis
We hypothesized that audio-motor transformation (AMT) play an important role in voice-guided movement initiation with STN involvement. We propose AMT-related subthalamic nucleus activation analysis in lateralized PD for tremor asymmetry influence study. We identified PD symptoms laterality dependent STN activation peculiarities. Obligatory left STN activation in AMT supposes its role in motor command switching. Bilateral STN activation during the movement execution supports its proposed role as a motor error correction node.
Introduction
Tremor asymmetry is considered a crucial criterion for PD and may influence future disease progression, concerning motor and non-motor symptoms development [Erro et al. 2012]. Subthalamic nucleus (STN) gained increased attention in PD due to its role in tremor origin and brain stimulation treatment [Helmich, 2012]. We hypothesized that audio-motor transformation (AMT) play an important role in voice-guided movement initiation with STN involvement. We propose AMT-related STN activation analysis in lateralized PD for tremor asymmetry influence study.Methods
Three groups of right-handed subjects (G1, G2, G3) were studied by fMRI with 1.5T SIGNA EXCITE (GE, USA). G1 consisted of 7 healthy subjects (4F, 51-83 y.o.). G2 consisted of 6 non-demented (MoCA 25-27) PD patients with left-side (non-primary hand) tremor (2F, 53-74 y.o.). G3 consisted of 5 non-demented (MoCA 25-30) PD patients with right-side (primary hand) tremor (2F, 56-74 y.o.). For G2/G3 disease duration was 2-8 years, Hoehn-Yahr scale 2.5-3. Simple finger tapping task was used for fMRI activation. Measured simple reaction time in G1/G2/G3=302/352/328 ms. Brain activation during the AMT phase (voice command assisted movement onset/stop) and movement execution phase was analyzed. Single shot GE-EPI sequence was used for BOLD imaging (TR/TE=3000/71 ms, voxel=4x4x5 mm). Anatomical images were acquired with FSPGR sequence (TR/TE=11.6/5.2 ms, TI=450 ms, voxel=1x1x1.5 mm). FMRI data processing was carried out using GLM (FEAT) and ICA (MELODIC) based software from FSL (Oxford, GB). Pre-processing, including motion correction, slice timing correction, spatial smoothing (FWHM=8 mm) was done. Single subject and group GLM and ICA analyses were done.Results
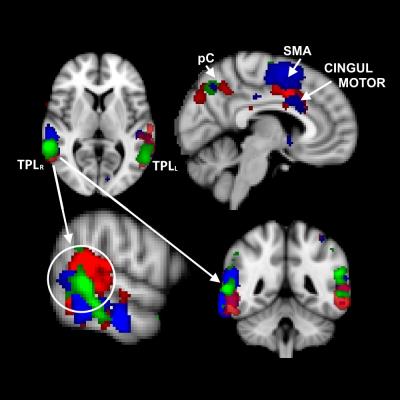
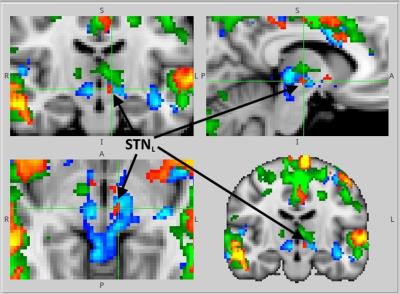
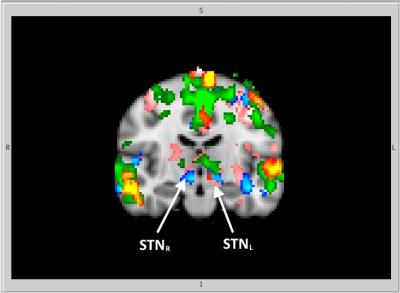
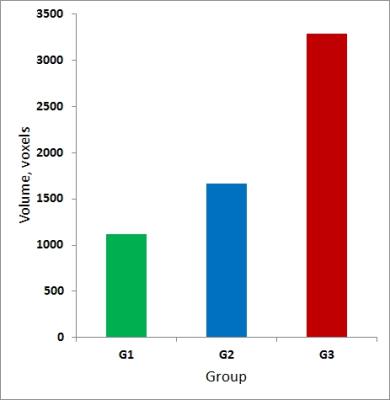
GLM and ICA-based analyses of brain activation during AMT for G1, G2, G3 revealed activation of the bilateral posterior temporal lobe (TPLL, TPLR), precuneus (pC), supplementary motor area (SMA) and cingulate motor region (Cingul. Motor) (Fig.1). Left STN activation during AMT was shown for G1, G2, G3 (Fig.2). Bilateral activation of STN for G2 during AMT was shown (Fig.2). Specific bilateral activation of STN during the movement execution phase (additional to the left STN activation during the AMT) was shown for G3 (Fig.3). The increased total volume of activation was shown for G3 in comparison to G2 and G1 (Fig.4). The process of voice-guided movement execution and AMT in PD patients and age-matched subjects evokes activation of auditory, sensory-motor cortex and an important node of default mode network (pC) with no impact of tremor laterality. However, tremor laterality influences STN activation greatly. While left STN was shown to be activated exceptionally during AMT in healthy subjects and PD patients, in right side tremor PD patients bilateral STN activates during all the movement execution. While STN was shown to correlate with tremor laterality and occurrence [Helmich, 2012], also it was shown to participate in movement error detection [Tan, 2014] and to function as a central component of the arbitrary system of the basal ganglia [Servestani, 2011]. Specific activation of bilateral STN during the movement execution in primary side affected PD patients, together with the increased total volume of activation and quite shorter simple reaction time suppose STN compensative and corrective role in affected side movement.Conclusion
We identified PD symptoms laterality dependent STN activation peculiarities. Obligatory left STN activation in AMT supposes its role in motor command switching. Bilateral STN activation during the movement execution supports its proposed role as a motor error correction node. AMT-related STN activation might be used as a potential probe for future PD studies.Acknowledgements
No acknowledgement found.References
1. Erro, R., Santangelo, G., Picillo, M., et al. (2012), ‘Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients’, Journal of neurology, vol. 259, no. 9, pp. 1808-1813.
2. Helmich, R. C., Hallett, M., Deuschl, G., et al. (2012), ‘Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits?’, Brain, vol. 135, no. 11, pp. 3206-3226.
3. Tan, H., Zavala, B., Pogosyan, A., et al. (2014), ‘Human Subthalamic Nucleus in Movement Error Detection and Its Evaluation during Visuomotor Adaptation’, The Journal of Neuroscience, vol. 34, no. 50, pp. 16744-16754.
4. Sarvestani, I. K., Lindahl, M., Hellgren-Kotaleski, J., & Ekeberg, Ö. (2011). ‘The arbitration–extension hypothesis: a hierarchical interpretation of the functional organization of the basal ganglia’, Frontiers in systems neuroscience, vol. 5.