5282
Accelerated intermittent theta burst stimulation, applied to the left DLPFC, influences dynamics in depression related networks1Eindhoven University of Technology, Eindhoven, Netherlands, 2University Hospital Ghent, Ghent, Belgium, 3Academic Center for epileptology Kempenhaeghe, Heeze, Netherlands
Synopsis
The effect of accelerated intermittent theta burst stimulation (aiTBS) is investigated in three resting-state networks involved in depression: default mode network (DMN), central executive network (CEN), and salience network (SN). Multivariate Granger causality analysis was performed between time-series representing each network and between time-series of nodes belonging to these networks. The effects of the latter analysis were quantified by the in- and out-degree. No between-network effects were found but specific connections showed increased or decreased Granger causality after stimulation. Clinical responders showed changes in the in- and out-degree of the anterior cingulate, known to be important in depression pathology.
PURPOSE
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive neurostimulation technique that has shown promising results in the treatment of various neuropsychiatric disorders. Major depressive disorder (MDD) patients are characterized by hypo-activity of the left dorsolateral prefrontal cortex (DLPFC). High frequency TMS treatment applied to this region, assumed to induce excitatory after-effects, is an FDA approved treatment for MDD. In this study, the effect of accelerated intermittent theta burst stimulation (aiTBS)1, a protocol in which multiple iTBS sessions2 are applied, is investigated in three resting-state networks known to be important in depression: default mode network (DMN), central executive network (CEN), and salience network (SN)3.METHODS: Acquisition
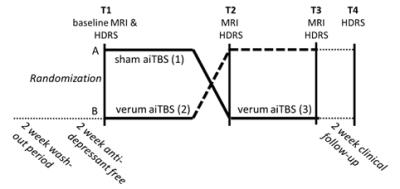
Fifty patients with MDD were included in a double-blind, randomized, sham-controlled, cross-over stimulation design. The left DLPFC was stimulated at 110% resting motor threshold in five sessions per day on four consecutive days (Magstim Super Rapid). One stimulation session contains 54 iTBS trains (10 bursts of 3 stimuli) adding up to 32,400 stimuli in total. Patients received both sham and verum iTBS. Anatomical and resting-state functional MRI (rs-fMRI) was recorded before (T1) stimulation and after sham and verum iTBS (T2/T3, Figure 1). At these time-points and at T4, two weeks after the final stimulation, clinical well-being was assessed using the Hamilton Depression Rating Scale (HDRS).METHODS: Between-network analysis
Data of 42 patients could be used for analysis. Pre-processing of the rs-fMRI data was performed (slice time correction, realignment, coregistration, normalization). Masks representing the DMN, CEN, and SN were taken from literature4,5. Time-series representing these networks were extracted by averaging the time-series of the voxels within the masks. The effect of aiTBS on dynamics was investigated by comparing Granger causality, calculated using the multivariatie Granger causality (MVGC)6 toolbox, between the networks.METHODS: Node analysis
To zoom in on specific connections, we repeated the MVGC analysis using the time-series of nodes that belong to either DMN, CEN, or SN. Nodes were defined by freesurfer’s Destrieux atlas, and selected for one of the subnetworks if the node had more than 25% overlap with the standard masks4,5. This resulted in 37 nodes belonging to either DMN, CEN or SN. For every node, the mean rs-fMRI time-series was derived by averaging over the voxels within the node. For every node, the weighted in-degree and out-degree were computed by summing over the Granger causality values to other nodes7.METHODS: Statistics
Repeated measures ANOVA was split into three parts comparing Granger causality values after baseline to sham values (1 in Figure 1), baseline versus verum values (2), and sham versus verum values (3), correcting for age and gender. Note that verum versus sham was not compared because of a potential cross-over effect of the stimulation. Significance level was set to p<0.05. For the between-network analysis, Bonferroni correction was applied to correct for multiple comparisons (pMVGC = 0.05/6). Statistical analysis was performed for all patients and for patients who showed clinical response after stimulation (n = 24)8. Clinical response was defined as an improvement (decrease) in HDRS between T1 and T4 of at least 7.RESULTS
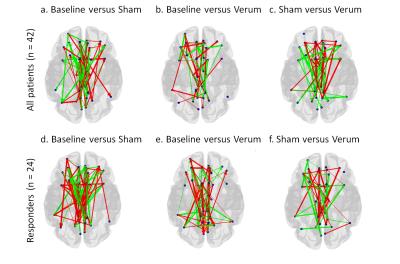
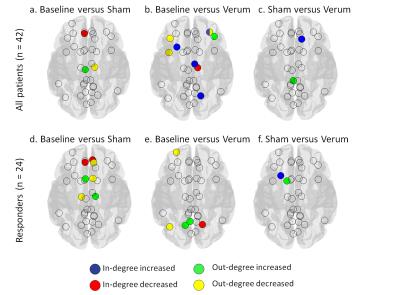
No effects of stimulation were found in the between-network analysis. However, node analysis showed significant effects of stimulation on Granger causality in some connections (Figure 2). Figure 3 shows the significant effects of in- and out-degree, based on Granger causality, for all patients and the subgroup of responders respectively.
DISCUSSION
As can be seen in Figures 2 and 3, the effects of stimulation are different between all patients and the subgroup of responders (n=24). These differences might, at least partly, be explained by a difference in sample size. There is an effect of sham stimulation. This is in concordance with the clinical findings after sham stimulation of Duprat et al.1. Especially the changes in in-degree and out-degree in the responder group involve the middle and anterior cingulate, a structure known to be highly involved in depression pathology9. Comparing baseline to verum stimulation showed decreased in-degree in the right parieto-occipital sulcus, which is known to be connected to the left DLPFC, where we stimulated. The additional effects on in-degree and out-degree based on Granger causality after verum compared to sham stimulation seem to be limited. This indicates a possible placebo effect of sham stimulation.CONCLUSION
In this study, no changes in the dynamics between DMN, CEN, and SN were found after application of aiTBS. However, specific connections within these three networks have shown to be influenced by aiTBS that might link to attenuation of depression symptoms. More knowledge about brain networks, and their interaction, is necessary to understand the effect of aiTBS and clinical effects in more detail.Acknowledgements
No acknowledgement found.References
1. Duprat R., Desmyter S., De Raedt R., Van Heeringen K., Van den Abbeele D., Tandt H., Bakic J., Pourtois G., Dedoncker J., Vervaet M., Van Autreve S., Lemmens G.M.D., Baeken C., Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: A fast road to remission? Journal of Affective Disorders 2016; 200: 6-14
2. Huang Y.Z., Edwards M.J., Rounis E., Bhatia K.P., Rothwell J.C., Theta burst stimulation of the human motor cortex. Neuron 2005; 45(2): 201-206
3. Sridharan D., Levitin D.J., Menon V., A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. PNAS 2008; 105(34):12569-12574
4. Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F., Correspondence of the brain’s functional architecture during activation and rest. PNAS 2009; 106(31):13040-13045
5. Menon V., Salience network. Brain Mapping: An encyclopedic reference 2008; 2:597-611
6. Barnett L., and Seth A.K., The MVGC multivariate Granger causality toolbox: A new approach to Granger-causal interference. Journal of Neuroscience Methods 2014; 223:50-68
7. Rubinov M. and Sporns O., Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010; 52: 1059-1069
8. Nettekoven C., Volz L.J., Leimbach M., Pool E., Rehme A.K., Eickhoff S.B., Fink G.R., Grefkes C., Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. NeuroImage 2015; 118: 209-218
9. Fox M.D., Buckner R.L., White M.P., Greicius M.D., Pascual-Leone A., Efficacy of TMS targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry 2012; 72(7): 595-603
Figures