5281
The GABA Level in DMN Modulate the Brain Network Centrality1Department of Radiology, Taipei Veterans General Hospistal, Taipei, Taiwan, 2Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, 3GE Healthcare, Taipei, Taiwan, 4GE Healthcare MR Research China, Beijing, People's Republic of China, 5GE Healthcare, Berlin, Germany, 6Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan
Synopsis
In this study, we combined resting-state functional magnetic resonance imaging (rsfMRI) and advanced magnetic resonance spectroscopy technique to demonstrate a positive relationship between levels of inhibitory neurotransmitter gamma-aminobutyric acid (GABA) within posterior cingulate cortex/precuneus (PCC/PCu) and high network centrality of primary network. High network centrality propagates and contributes to efficient information flow in brain network. The PCC/PCu is a key component of default mode network (DMN) and high regional GABA levels expressing in the PCC/PCu area deactivate DMN activities related to internal thoughts for reallocating attention resources from internal processes to goal directed external stimuli with high network centrality.
Purpose:
At the cellular level, multiple neurochemical processes regulate neuronal activity. Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitters in the brain. GABAergic interneurons are believed to have a direct impact on blood oxygen level dependent (BOLD) signal1 and BOLD signal has been found to be correlated with brain neural activity2. In resting state, the major activities of brain area are in posterior cingulate cortex/precuneus (PCC/PCu), medial prefrontal cortex (mPFC), and hippocampus. These areas were defined as default mode network (DMN) which is consistently deactivation during the tasks requiring external orientation3 and thought to be associated with suppression of spontaneous brain activities and reallocation of brain resources for ongoing, attention-demanding tasks4. Functional network centrality is a key concept in brain network analysis and serves as a hub for superior information propagation and contributes to efficient information flow in brain network. By combining BOLD-weighted rsfMRI and advanced magnetic resonance spectroscopy (MRS) techniques, the relationship between neurotransmitters and brain network hubs modulation can be examined at a system level. We applied centrality node analysis to the study of rsfMRI data of the human brain in healthy subjects with GABA level measurements in PCC/PCu by spectral-editing Mescher–Garwood point-resolved spectroscopy (MEGA-PRESS) sequence. Within a global scale analysis of the brain network in a graph theory approach, we hypothesized that the GABA suppress the spontaneous brain activities in DMN and would reallocate the resources to high network centralities of primary network for input ongoing, attention-demanding taskMethods:
Twelve healthy subjects (age: 29.6.4 ± 2.1 years; 6 females) participated in the study. MRS and fMRI scans were performed on a 3T clinical scanner (Discovery MR750, GE Healthcare, Milwaukee, USA) using a body coil as RF excitation and an 8-chanel head coil as signal receiver. A 3×3 ×3 cm3 voxel of interest (VOI) was placed at the PCC/PCu (Figure 1A). The 1H spectrum optimized for detecting GABA was acquired by a MEGA-PRESS sequence with the following parameters: TE/TR = 68/1500 ms; number of points= 2048; spectral width =2000 Hz; number of average = 160 (scan time = 11.92 min). A single-shot gradient-echo echo-planar imaging sequence was used to acquire BOLD images during resting-state. The imaging parameters were as follows: TR/TE = 2500/30 ms; FA = 78°; slice thickness = 3.5 mm without gap; 43 slices; FOV= 224 × 224mm2 with in-plane resolution of 3.5 × 3.5 mm2. GABA concentration was calculated using the GABA Analysis Toolkit (Gannet, http://gabamrs.org), which uses a Gaussian baseline model to fit the edited GABA signal and a Lorentz-Gaussian lineshape to fit the unsuppressed water signal. Processing for rsfMRI data were used DPARSFA toolbox5, the steps included slice-timing correction, head motion correction, spatial smoothing with 6 mm Gaussian kernel, detrending filter (0.08-0.1 Hz) and then normalized to standard MNI space with a resampling resolution of 3 × 3 ×3 mm3. The six motion parameters, CSF and WM signal were also regressed out. Network centrality map was calculated by voxel-wise time course correlation at > 0.25 and were converted into z-score map before further parametric statistical analysis. A correlation between of GABA level in PCC/PCu and node centrality within groups was then assessed using simple regression with SPM and corrected for multiple comparisons using a combination of an uncorrected height threshold of p < 0.01 with a minimum cluster size 50. The cluster size was determined over 1000 Monte Carlo simulations using AlphaSim program.Results:
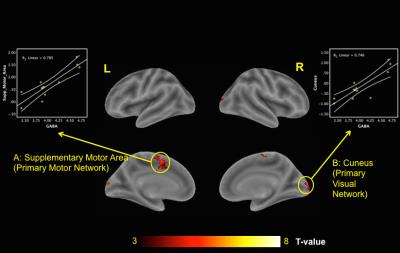
Representative profile of GABA MRS generated by MEGA-PRESS sequence and the fitting results of GABA signal using GANNET toolbox are showed in Figure 1B and Figure 1C, respectively. The GABA peak at 3.0 ppm can be well visualized and minimal residual signal was found which expresses the good fit of GABA. The whole brain node centrality highly significant correlated with GABA level in PCC/PCu are showed in Figure 2. The highly connected network centralities in whole brain are supplementary motor area (primary motor network) and Cuneus (primary visual network) are significantly correlated with the increase of GABA levels in PCC/PCu.Discussion and Conclusion:
Our results demonstrate that high GABA level in PCC/PCu are significant association with high network centralities in primary network; which are visual and motor network. Highly network centrality propagates and contributes to efficient information flow in brain network. The high GABA level expressing in PCC/PCu modulate DMN deactivation and consequently suppress ongoing brain activities related to internal thoughts for reallocating attention resources from internal processes to goal directed external stimuli with high centrality in primary network. In conclusion, the inhibitory neurotransmitter, GABA, modulate the brain network centralities by deactivating BOLD signal in DMN.Acknowledgements
No acknowledgement found.References
1. G. Buzsáki, K. Kaila, M. Raichle, Inhibition and brain work, Neuron, 56 (2007) 771-783.
2. N.K. Logothetis, J. Pauls, M. Augath, T. Trinath, A. Oeltermann, Neurophysiological investigation of the basis of the fMRI signal, Nature, 412 (2001) 150-157.
3. G.L. Shulman, J.A. Fiez, M. Corbetta, R.L. Buckner, F.M. Miezin, M.E. Raichle, S.E. Petersen, Common blood flow changes across visual tasks: II. Decreases in cerebral cortex, Journal of cognitive neuroscience, 9 (1997) 648-663.
4. K.A. Mckiernan, J.N. Kaufman, J. Kucera-Thompson, J.R. Binder, A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging, Journal of cognitive neuroscience, 15 (2003) 394-408.
5. Y. Chao-Gan, Z. Yu-Feng, DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI, Frontiers in systems neuroscience, 4 (2010).
Figures