5275
Dynamic Changes of Functional Connectivity within and between Resting State Networks in Intractable Mesial Temporal Lobe EpilepsyChao Zhang1, Kuncheng Li1, Nan Chen1, and Hongyu Yang1
1Xuanwu Hospital,Capital Medical University, Beijing, People's Republic of China
Synopsis
This study aimed to observe dynamic functional organization changes of large-scale resting state network (RSN) in MTLE and the patient who got seizure free after surgical treatment. Subject specific RSNs of three groups (healthy controls, presurgical group and posttreatment group) were extracted using group-information guided independent component analysis. Then, we calculated and compared the FC results between three groups, and we found FC altered markedly before and after surgical treatment. In addition, there was no statistical difference between posttreatment group and healthy controls. Our results may provide valuable information for further understanding of the pathophysiological mechanisms of intractable MTLE.
Introduction
Intractable mesial temporal lobe epilepsy (MTLE) is the most common epilepsy and most of which will receive surgical treatment. 1, 2 The concept of the whole brain network disorder of MTLE has been accepted as a result of many previous studies, 3-5 though its frequent pathological findings were limited in mesial temporal lobe structure. Many resting state functional MRI (rs-fMRI) studies have revealed resting-state networks (RSNs) aberrant in MTLE, 6-8 however, few studies have focused on dynamic changes of functional connectivity (FC) in RSNs before and after surgical treatment. Furthermore, the mechanism of epilepsy network is largely unknown now [9]. The aim of this study was to investigate dynamic changes of FC within and between RSNs in unilateral MTLE patients before and after surgical treatment.Method
Rs-fMRI data were acquired from 7 unilateral intractable MTLE patients (3 males, 4 females; age=26.86 ± 7.89, range=16 - 34 years) and all this 7 patients received surgical treatment. The control group included 18 healthy volunteers (10 males, 8 females; age=26.61 ± 2.89, range=23 - 35 years). Patients were divided into two groups: presurgical group and posttreatment group. The diagnosis and lateralization of the seizure focus were determined by a comprehensive evaluation in our epilepsy consultation center. None of the patient had a mass lesion (tumor, cortical, or vascular malformations) or traumatic brain injury. All patients were confirmed with hippocampal sclerosis (HS) though imaging examination and/or postoperative pathology studies, in addition, no patient got relapse after surgical treatment. Data were collected on a MAGNETOM Trio Tim 3T MR scanner (Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. The resting blood oxygen level-dependent images were acquired with echo-planar imaging sequence with the following parameters: TR/TE = 2000/30 ms, FOV = 220 mm × 220 mm, GRAPPA (PE) 2, slice thickness = 3 mm, voxel size = 3.4 mm × 3.4mm × 3.0 mm, 35 slices, flip angle = 90°, and total acquisition time = 6:08 min and 180 volumes. T1-weighted images were acquired using a 3D-MP-RAGE, providing isotropic voxels of 1mm × 1mm × 1 mm. The resting-state fMRI data of all subjects were preprocessing using Data Processing Assistant for Resting-State fMRI (DPARSF). Subject specific RSNs for three groups were estimated through group-information guided independent component analysis (GIG-ICA). 10 One-way ANOVA was used for analyzing intranetwork and internetwork FC differences among three groups. p<0.05 was considered statistically significant.Result
ECN (executive control network), SMN (sensorimotor network) and DMN (default mode network) were identified with significant FC changes using One-way ANOVA (p < 0.05, FDR corrected). Presurgical MTLE patients showed significantly increased FC between ECN and rSMN, as well as between DMN and SMN when compared with healthy controls (p < 0.05, FDR corrected) (Figure 1). The same extrinsic FCs were decreased significantly after surgical treatment when compared with presurgical group (p < 0.05, FDR corrected) (Figure 1). Additionally, posttreatment patients exhibited no significantly FC change within and between all RSNs when compared with healthy controls (p > 0.05, uncorrected). Compared with healthy controls, only presurgical MTLE patients exhibited intranetwork FC in the left ECN (p<0.05, GRF corrected) (Figure 2) and intra-DMN FC between left supramarginal and right inferior parietal lobule changes (p=0.0013, uncorrected) (Figure 1).Discussion
Previous studies have revealed aberrant FC within several RSNs in unilateral MTLE, such as DMN, AN and perceptual networks. 6, 11-13 Postoperation study also demonstrated dorsal DMN impaired in TLE. 7 Our result of FC in DMN was agreement with previous reports. However, they mainly focused on a single RSN which can’t represent the entire epileptic network. To the best of our knowledge, this is the first study that investigates functional organization dynamic changes of whole brain large-scale networks in unilateral intractable MTLE. We observed abnormal FC pattern between the ECN and the rSMN and between the rSMN and the DMN in MTLE. Additionally, such abnormal functional organization can be eliminated though successful surgical treatment. These findings not only confirmed MTLE a brain network disorder again, but also further imply that the abnormal internetwork FC of RSN may act as a potential biomarker of MTLE and provides insights into biological mechanism of the disease.Conclusion
Our study suggested that, the large-scale functional organization in presurgical patients may complement the characteristics of epileptic network in another way. Moreover, the dynamic changes of FC before and after surgical treatment may help us to further understand physiological mechanism of unilateral intractable MTLE.Acknowledgements
No acknowledgement found.References
1. Jobst B C, Cascino G D. Resective epilepsy surgery for drug-resistant focal epilepsy: a review. JAMA, 2015, 313(3): 285-293. 2. Ryvlin P, Cross J H, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol, 2014, 13(11): 1114-1126. 3. Zhang Z, Liao W, Chen H, et al. Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain, 2011, 134(Pt 10): 2912-2928. 4. Liu M, Bernhardt B C, Hong S J, et al. The superficial white matter in temporal lobe epilepsy: a key link between structural and functional network disruptions. Brain, 2016, 139(Pt 9): 2431-2440. 5. Voets N L, Beckmann C F, Cole D M, et al. Structural substrates for resting network disruption in temporal lobe epilepsy. Brain, 2012, 135(Pt 8): 2350-2357. 6. Zhang Z, Lu G, Zhong Y, et al. Impaired perceptual networks in temporal lobe epilepsy revealed by resting fMRI. J. Neurol., 2009, 256(10): 1705-1713. 7. Doucet G E, Skidmore C, Evans J, et al. Temporal lobe epilepsy and surgery selectively alter the dorsal, not the ventral, default-mode network. Front Neurol, 2014, 5: 23. 8. Cataldi M, Avoli M, De Villers-Sidani E. Resting state networks in temporal lobe epilepsy. Epilepsia, 2013, 54(12): 2048-2059. 9. Paz J T, Huguenard J R. Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat. Neurosci., 2015, 18(3): 351-359. 10. Du Y, Fan Y. Group information guided ICA for fMRI data analysis. Neuroimage, 2013, 69:157-197. 11. Li W, Chen Z, Yan N, et al. Temporal Lobe Epilepsy Alters Auditory-motor Integration For Voice Control. Sci Rep, 2016, 6:28909. 12. James G A, Tripathi S P, Ojemann J G, et al. Diminished default mode network recruitment of the hippocampus and parahippocampus in temporal lobe epilepsy. J Neurosurg, 2013, 119(2): 288-300. 13. Zhang Z, Lu G, Zhong Y, et al. Impaired attention network in temporal lobe epilepsy: a resting FMRI study. Neurosci. Lett., 2009, 458(3): 97-101.Figures
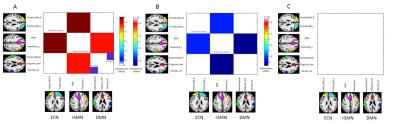
Figure
1. Functional
connectivity (FC) changes of extrinsic and intrinsic RSNs with significant
differences between three groups. A = presurgical patients vs healthy
controls; B = posttreatment vs presurgical patients;
C = posttreatment vs healthy controls; ECN, executive control network; SMN, sensorimotor network; DMN, default mode network.
Figure
2. Brain
region wtih significant changes in intranetwork FC in presurgical MTLE patients
(p<0.05, GRF corrected).