5264
Crossover Intra-individual Comparison Study of Non-ionic and Ionic Gadolinium Based Contrast Agents in the Quantitative Evaluation of C6 Glioma with DCE-MRI1PLA General Hospital, Beijing, People's Republic of China
Synopsis
In this crossover intra-individual comparison study, we aimed to compare the non-ionic (godadiamide, Gd-DTPA-BMA) and ionic (gadopentetate dimeglumine, Gd-DTPA) Gadolinium based contrast agents (GBCA) in the quantitative evaluation of C6 glioma with dynamic contrast enhanced MR imaging (DCE-MRI) at 3.0 T MR scanner. Ktrans, Ve and VP maps were generated. Three radiologists independently performed tumor segmentation and value calculation. Gd-DTPA-BMA has significant more pixel counts of glioma in Ktrans map and increased tendency for average Ktrans and Kep values, indicating that DCE-MRI with Gd-DTPA-BMA may be more suitable and sensitive for the evaluation of glioma.
Target audience
Dynamic contrast enhanced MR (DCE-MR) is a promising technique for tumor monitoring especially for tumor treatment evaluation. Different contrast media choice will result in different parameters even by using same model. All the MR Magnetic resonance technicians and doctors are the target audience of the study.Objective
This study aimed to prospectively compare the non-ionic (godadiamide, Gd-DTPA-BMA) and ionic (gadopentetate dimeglumine, Gd-DTPA) Gadolinium based contrast agents (GBCA) for dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) of C6 glioma at 3.0 TeslaMethods
C6 glioma model was established in 18 Wistar rats. DCE-MRI was performed 6 days after tumor implantation with MR (3.0 T, Discovery 750, GE healthcare, Milwaukee, USA) scans. Each rat received two identical DCE-MRI scans with Gd-DTPA-BMA and Gd-DTPA separated by 24 hours in randomized order. Scanning protocol was VIBRANT, including T1map (TR 7.4 ms, TE 2.0 ms, slice gap=3, slice gap=0, matrix=160×128, FOV=12cm, Flip angle=2 o, 3 o, 6 o, 9 o, 12 o, 15 o) and dynamic enhanced T1WI (Flip angle=12 o, the other patameters are the same as T1map) after a bolus injection (0.2ml/s) at 0.4 mmol/kg. Image data were processed with two-compartment model. Ktrans, Ve and VP maps were generated, and the tumors were manually segmented on all three dimensional (3D) maps. Kep values were calculated with the values of Ktrans and Ve. Pixel counts and mean values were recorded for paired-t test. Three radiologists who were blind to the groups independently performed tumor segmentation and value calculation. The agreements from different observers were subjective to intra-class correlation coefficient (ICC).Results
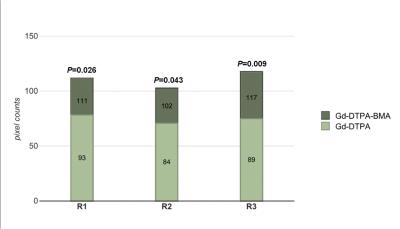
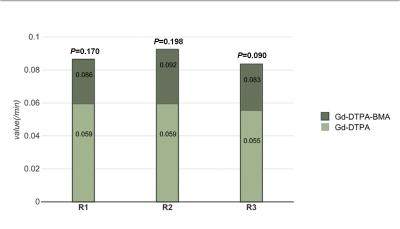
Three readers demonstrated the pixel counts of tumor in Ktrans map were higher with Gd-DTPA-BMA than the counts with Gd-DTPA (P= 0.026, P=0.043, P=0.009). Although the Ktrans and Kep values were higher with Gd-DTPA-BMA than with Gd-DTPA, there were no statistical significance (P>0.05, all readers), respectively. The pixel counts and values of tumor with Gd-DTPA-BMA and Gd-DTPA in Ve map and Vp map were similar (P>0.05, all readers). Inter-observer agreement was excellent for all assessments (ICC = 0.802-0.983).Discussion
It was demonstrated in our study that DCE-MRI apparently captures more leaky pixels by using Gd-DTPA-BMA than Gd-DTPA, suggesting that non-ionic GBCA with low osmolarity, low viscosity, and low molecular weight could penetrate the broken BBB easier and reveal more permeable location in the vascular wall of the tumor. Ktrans and Kep values showed an increased tendency with Gd-DTPA-BMA, but there were no difference between the two GBCAs on Ve and Vp maps since Ve and Vp represent the density of contrast agent saved in extracellular fluid and vascular space, which are not affected by the permeability.Conclusion
Different GBCAs can potentially influence DCE-MRI data at 3.0 T. More leaky pixel were found with Gd-DTPA-BMA in C6 glioma model by using DCE-MRI compared with Gd-DTPA.Acknowledgements
No acknowledgement found.References
References
1. Gordon Y, Partovi S, Müller-Eschner M. Dynamic contrast-enhanced magnetic resonance imaging: fundamentals and application to the evaluation of the peripheral perfusion. Cardiovasc Diagn Ther. 2014;4:147-164.
2. Bellin MF, van der Molen AJ. Extracellular gadolinium-based contrast media: an overview. Eur J Radiol. 2008;66:van der Molen AJ, Bellin MF. Extracellular gadolinium-based contrast media: differences in diagnostic efficacy. Eur J Radiol. 2008;66:160-167.
3. van der Molen AJ, Bellin MF. Extracellular gadolinium-based contrast media: differences in diagnostic efficacy. Eur J Radiol. 2008;66:168-174.
4. Quick A, Patel D, Hadziahmetovic M, et al. Current therapeutic paradigms in glioblastoma. Rev Recent Clin Trials. 2010;5:14-27.
5. Allhenn D, Boushehri MA, Lamprecht A. Drug delivery strategies for the treatment of malignant gliomas. Int J Pharmaceut. 2012;436:299-310.
6. Jensen RL, Mumert ML, Gillespie DL, et al. Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol. 2014;16:280-291.
Figures