5262
A Robust non-balanced SSFP fMRI Technique for High Field: Comparison with SE-EPI and bSSFP Techniques at 7 Tesla1Biomedical Engineering, Amirkabir University of Technology (Tehran Polytechnic), Tehran, Iran, 2Donders Institute for Brain, Cognition and Behaviour, Radboud university Nijmegen, Nijmegen, Netherlands, 3School of Cognitive Sciences, Institute for Research in Fundamental Sciences (IPM), Tehran, Iran
Synopsis
T2-weighted fMRI methods including Spin-Echo (SE) and balanced SSFP (bSSFP), became of particular interest to reach superior functional specificity in high field application. However, both techniques suffer from a number of practical limitations. SE fMRI may be SAR limited at high fields. On the other hand, bSSFP suffers from dark bands. To eliminate dark bands in bSSFP, non-balanced-SSFP (nbSSFP) fMRI was previously suggested. Here, we developed a robust version of nbSSFP by using a single SSFP-echo sequence to decrease motion effects. The performance of the suggested sequence is evaluated and the results obtained are compared with bSSFP and SE-EPI fMRI.
Purpose
T2-weighted fMRI methods including Spin-Echo (SE) and balanced SSFP (bSSFP), became of particular interest to reach superior functional specificity in high spatial resolution applications. Several studies have demonstrated that utilizing higher static field increases the functional signal localization [1-3] with these techniques compared to T2* weighted methods. However, both SE and bSSFP suffer from a number of practical limitations. SE fMRI may be SAR limited at high fields [4]. On the other hand, bSSFP shows a dramatic sensitivity to off-resonance frequency which can manifest as dark bands which are further exacerbated at higher magnetic fields [5]. To get rid of dark band artifacts in bSSFP, non-balanced-SSFP (nbSSFP) fMRI was previously suggested [6-7]. Here, we developed a robust and efficient version of nbSSFP by using a single SSFP-echo sequence (also known as S2 signal) to decrease motion effects. The performance of the suggested sequence is evaluated and the results obtained are compared with bSSFP and SE-EPI fMRI.Method
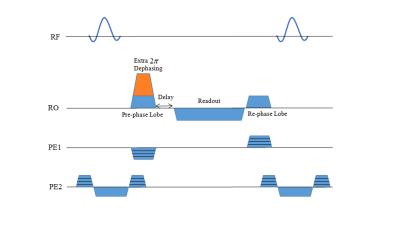
In this study, we developed an efficient SSFP sequence to capture the SSFP-echo signal which is more similar to SE signal by adding an extra 2π de-phasing gradient at the start of each TR followed by a delay prior to the readout gradient, to both nullify the first gradient moment for the primary spin echo (Fig. 1) and eliminate the FID-like (S1) signal. Task related fMRI experiments were performed utilizing a checker-board alongside with right hand finger tapping performed simultaneously from 7 healthy subjects on a 7 Tesla Siemens scanner. Implemented parameters were as follows: FA=23, TR=14.1 ms, TE=10.9 ms, voxel size=1.25x1.25x5 mm3, Matrix size=160x148x12, Volume-TR=8s. To compare the results, for 2 subjects, experiments were accompanied by a similar bSSFP acquisition, with matched imaging parameters. SE-EPI was also acquired for those two subjects using the same FOV and matrix size with TE=67 ms and volume-TR=2.67 s. Total duration for all techniques was 320 s. All the processing steps including brain extraction, motion correction and GLM analysis steps were performed using FSL (FMRIB, Oxford Univ.). No spatial smoothing was used. For generating activation maps, cluster based thresholding with Z-score=2.3 and p=0.05 was applied.Results
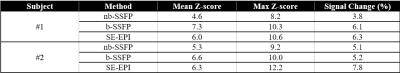
Two sample slices of a representative subject for proposed nbSSFP method along with bSSFP and SE-EPI imaging techniques are shown in Fig. 2. As can be seen here, bSSFP suffers from banding artifact and intensity variations due to its off resonance sensitivity. SE-EPI images show no banding but undergo geometrical distortions. In contrast, the nbSSFP images are free from both artifacts. In our nbSSFP results and by averaging across subjects, we found a mean z-score of 5.13 and a mean signal change of 4.67% for the 1000 voxels with the highest z-scores. Additionally, Fig. 3 shows the activation maps for one of the two subjects in which nbSSFP, bSSFP and SE-EPI have all been acquired. It can be seen that all three methods showed activation in the expected brain regions related to task. Although bSSFP, as expected, clearly suffers from dark bands. Table 1 shows a summary of the statistical results of both subjects for all three methods for the 1000 voxels with the highest z-scores. This table suggests slightly lower mean z-score and signal change for nbSSFP compared to SE-EPI and bSSFP.Discussion & Conclusion
The main advantage of the proposed technique is the high functional specificity, derived from T2-weighting, without banding artefact by utilizing SSFP-echo signal in the most efficient way to minimize flow and motion artefacts. Our approach for using nbSSFP was quite efficient as we focused on optimization of dephasing gradient value by decreasing TR and nulling the first moment. In comparison with other T2-weighted techniques, this sequence showed advantages over SE-EPI and bSSFP fMRI techniques in some aspects. The proposed technique with the mentioned parameters has nearly 28 times lower SAR compared to SE-EPI. Another limitation of SE-EPI is related to geometrical distortion due to EPI readout (Fig. 2). bSSFP is the other T2-weighted technique for higher functional specificity and low SAR, but suffers from banding artifact. According to Fig. 3, false positive rate increases substantially in their extended vicinity due to high sensitivity to off-resonance effects. This is in part responsible for the higher z-scores for bSSFP studies. In conclusion, our preliminary data show consistent reliable results with functional contrast comparable to bSSFP and SE-EPI fMRI but with no banding artifact, SAR problem or significant geometrical distortions.Acknowledgements
This study is partially supported by Cognitive Sciences and Technologies Council, Tehran, Iran (No. 2137 and No. 2603).References
1. Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn. Reson. Med. 1995;34:555–566.
2. Uludag K, Müller-Bierl B, Ugurbil K. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage 2009;48:150–165.
3. Norris DG. Spin-echo fMRI: the poor relation? Neuroimage 2012;62:1109–1115.
4. Norris DG, Koopmans PJ, Boyacioglu R, Barth M. Power independent of number of slices (PINS) radiofrequency pulses for low-power simultaneous multislice excitation. Magn. Reson. Med. 2011;66:1234–1240.
5. Scheffler K, Ehses P. High-resolution mapping of neuronal activation with balanced SSFP at 9.4 tesla. Magn. Reson. Med. 2015.
6. Auerbach EJ, Heberlein K, Hu X. High-resolution T2 fMRI at high magnetic fields using PSIF. In: Proceedings of the 10th Annual Meeting of ISMRM. ; 2002. p. 123.
7. Barth M, Meyer H, Kannengiesser SAR, Polimeni JR, Wald LL, Norris DG. T2-weighted 3D fMRI using S2-SSFP at 7 tesla. Magn. Reson. Med. 2010;63:1015–1020.
Figures