5250
Increased BOLD activation with high degree spherical harmonic shimming at 7TTae Kim1, Yoojin Lee1, Tiejun Zhao2, Hoby P Hetherington1, and Jullie W Pan1
1University of Pittsburgh, Pittsburgh, PA, United States, 2Siemens, Pittsburgh, PA, United States
Synopsis
B0 shimming is important for gradient echo functional MRI where T2* signal loss and precession occur from both physiological and non-physiological susceptibility effects. We used the high degree shim insert (3rd, 4th and two 5th degree shims) to assess its effect for 2mm isotropic whole brain GE-EPI BOLD (breath-hold induced) signal at 7T. Comparing 1st-2nd with high degree shimming, the Δ|B0| changes are spatially varying. For activation, the largest regions of increase are in the inferior frontal region; the largest regions of decrease are in the middle temporal lobe. Overall, there is a 4.3% increase in total activated pixels.
Purpose
The improvement of B0 shimming is important for gradient echo functional MRI where T2* signal loss and precession occur from both physiological and non-physiological susceptibility effects, these effects being substantially worse at 7T and with echo-planar imaging where pixel shift produces complex signal distortion. As a result, there has been much effort on methods to improve GE fMRI. Here we describe the quantitative improvement in whole brain BOLD signal and activated pixel counts using high degree spherical harmonic shimming.Methods
B0 shim insert, field map and shimming: A shim insert coil consisting of 3rd, 4th degree and two 5th degree shims (ZC4, ZS4) with 10A shim supplies (RRI, Billerica, MA) was used for high degree shimming, denoted as 1st-4th+ shimming. The B0 field was mapped using the B0 loop encoded readout (Bolero) method, with shimming performed by a least squares optimization based on a calibrated spherical harmonic model with a user-determined target ROI shown in Fig. 1A. To analyze EPI data, all pixels were binned in 10Hz increments according to change in |B0| offset between 1st – 2nd and 1st – 4th+ shims, i.e., ΔB0=|B01,2| - |B01-4+| (improved homogeneity pixels with 1st-4th+ compared to 1st-2nd shim are in positive bins; deteriorated pixels in negative bins). RF coil: An 8x2 (two rows, eight coils/row) inductively decoupled elliptical transceiver array was used in parallel transmit mode. RF shimming per subject was performed with eight 1kW transmit channels which were split and phased to generate a circularly polarized mode-like distribution. The achieved B1+ was 460±51Hz over the entire head at 1140±113 Watts total applied power. EPI and breath hold BOLD activation: Single-shot GE-EPI 2mm3 (48 slices, matrix size=96´96, bandwidth/pixel=13.88Hz, echospacing 750ms) was acquired in 6 volunteers; FOV=19.2x19.2cm2, TR=3.5s, TE=24ms, phase partial Fourier=6/8, FA=60°. For the whole brain BOLD signal response, a 21-sec breath-hold protocol was performed five times using a block-design paradigm (140s − [21s (breath hold) −35s] x5). N=8 subjects were asked to exhale prior to breath-holding to preclude a biphasic change (1). Each run was acquired with 1st-2nd and 1st-4th+ degree shimming and signal averaged in randomized order. Processing was performed using AFNI (http://afni.nimh.nih.gov/afni/), FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and SPM (http://www.fil.ion.ucl.ac.uk/spm/) programs combined with in-house programs written in Matlab R2013b. The Bolero B0 map was used to calculate inhomogeneity induced voxel displacements. Following normalization by the mean signal intensity at each voxel, BOLD maps were estimated by linear regression using AFNI (3dDeconvolve), which also included motion parameters. The significance of activation was determined on a pixel-by-pixel basis over each DB0 bin(p-value<0.05). MP2RAGE images were used for anatomical registration using (0.75 mm)3 resolution with TR=6s, TE=3.1ms, TI=0.8/2.7s, and GRAPPA 3. All studies were approved per the University of Pittsburgh IRB.Results
The ΔB0 bins, as presented in Talairach space from n=8 volunteers (Fig. 1B) shows that the high degree shimming had its greatest effects over the inferior frontal and temporal regions. For both the positive and negative bins, the BOLD % signal change is significant, being increased in the positive bins and decreased in the negative bins. In both positive and negative bins, there are significant changes in numbers of activated pixels. From n=8 volunteers, Fig. 2A shows that the high degree shimming significantly increased the numbers of activated pixels in the positive Δ|B0| bins, particularly above +40Hz, while they decreased in the negative bins. For all bins, the activated pixel numbers (green) were significantly different between 1st-2nd and 1st-4th+ shims. For most of the bins, the BOLD %signal change (orange) was significantly different (p<0.05) between the 1st-2nd and 1st-4th+ shims; Fig. 2B shows the locations and fractional change in activated pixel numbers and BOLD % signal change averaged over the 8 volunteers shown in Talairach space. Data from a single subject is shown in Fig. 2C.Discussion
As an independent means of improving field homogeneity, high degree shimming does not interact with other imaging parameters and is compatible with whole brain coverage necessary for simultaneous multi-slice methods. Comparing 1st-2nd and 1st-4th+ shimming, the Δ|B0| changes spatially vary over the brain with the majority of the brain either maintaining or increasing signal intensity and activation. Focusing on the pixels that substantially change (either improved or worsened, outside of the two center Δ|B0| bins) with the 1st-4th+ shimming, for each 1 activated pixel that is lost, there are 7.9 activated pixels gained. The largest regions of activation increase are seen in the inferior frontal region while the largest regions of activation decrease are in the middle temporal lobe. Overall, there is a 4.3% increase in total activated pixels.Acknowledgements
This work is supported by NIH R01EB011639; R01NS081772; R01NS090417; R01EB009871 and RSNA RSCH1314.References
1. Li TQ, Kastrup A, Takahashi AM, Moseley ME. Functional MRI of human brain during breath holding by BOLD and FAIR techniques. Neuroimage 1999;9(2):243-249.Figures
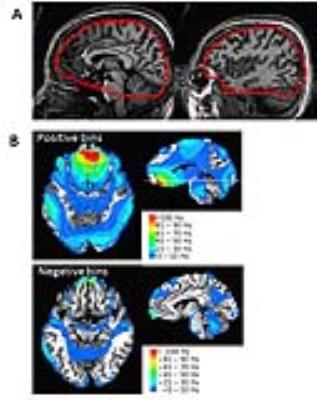
Fig. 1. (A) Typical VOI used for B0 shimming, shown in two sagittal-planes. The VOI
encompassed the temporal lobes and covered a longitudinal span of 96 mm. (B) From n=8 subjects, the regions of Δ|B0| =|B01-2|-|B01-4+|
bins (i.e., showing the regions of greatest change in B0 shimming) are
co-registered in Talairach space and overlaid on the T1-weighted image. As binned in 10Hz increments,
the positive Δ|B0| bins(top) and negative Δ|B0| bins(bottom) show
regions where 1st-4th+ is better or worse than 1st-2nd
degree shim respectively. Color bars: 10Hz |B0| bins. The volume of positive bins is much larger
than negative bins.
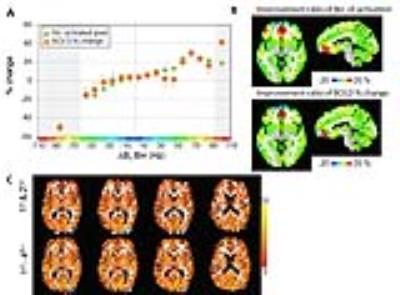
(A) The number of activated pixels and BOLD %change; the bins that were non-significant (BOLD %signal change) are marked by x. (B) The regional location of each ∆|B0| bin is shown in color according to the fractional change in either activated pixel number or BOLD% signal change as defined from panel (A). (C) BOLD activation maps are shown from a single subject: maps from 1st-2nd (top) and 1st-4th+ shimming (bottom) are overlaid on the forward warped T1-weighted images (using the Bolero B0 maps). The t-value is shown in the color scale.