5248
Poloxamer: a new means to recover functional network information in the rodent’s deep brain structures1Institute for Biomedical Engineering, ETH and University Zurich, Zurich, Switzerland, 2Roche Pharma Research & Early Development, Neuroscience Discovery, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd, Basel, Switzerland, 3Neuroscience Center Zurich, Zurich, Switzerland, 4Institute of Pharmacology and Toxicology, University of Zurich, Zurich, Switzerland
Synopsis
In the past years, functional MRI studies in rodents have become more frequent. The signal losses in gradient-echo EPI due to the increased sensitivity to magnetic susceptibility differences at higher magnetic field, however, make fMRI studies of deep brain structures difficult. Here we propose the use of Poloxamer, a non-toxic amphiphilic triblock copolymer well known for its thermo-reversible properties and pharmaceutical application to fill up the air cavities in the rodent middle and external ear canals. This practical method considerably increases geometric fidelity of the functional images, opening up the possibility to longitudinally investigate rodent’s deep brain networks.
Introduction
fMRI studies in rodents have mainly adopted the same experimental parameters as typically used in human fMRI. However, the higher magnetic fields used for rodent studies lead to stronger susceptibility artefacts. These signal voids, which are mainly observed at the air-tissue interface between the ear and the brain, result from intravoxel dephasing effects and make the study of deep brain structures difficult, if not impossible. The impact of such susceptibility artefacts can be somewhat reduced by resorting to shorter echo times and thinner slices, however at the cost of a reduced BOLD sensitivity and SNR, respectively. To overcome these susceptibility artefacts, researchers have attempted to fill the ear canals with susceptibility-matched substances such as tooth paste1 or Fomblin2. Nevertheless, besides the complexity of these protocols, they are either insufficient at 9.4T or are not stable over a prolonged time. Poloxamers are non-toxic3, non-irritant amphiphilic triblock copolymers with a surprising thermo-reversible gel formation; they are used for instance as excipients in the cosmetic and pharmaceutical industry4,5. Poloxamer solutions can be designed such that the transition temperature from liquid to gel occurs at the physiological temperature of 37 ºC. The solution thus is liquid at room temperature and can easily be injected into the ear canal; then reaching body temperature, it jellifies and remains stable within the animal, thus greatly simplifying administration procedures. In this work, we propose to use Poloxamer in the ear canals as a practical protocol paving the way for longitudinal fMRI studies of deep brain structures in rodents.Material and Methods
Studies were conducted on 4 Lewis rats (female, 180-200g) and were carried out on a BioSpec 9.4T MR system (Bruker BioSpin MRI, Germany) equipped with a volume resonator for transmission and a room temperature surface coil for reception. 20% (w/w) Poloxamer 407 (Sigma-Aldrich, Germany) solution was prepared in PBS by using the cold method4 to reach a transition temperature around 30ºC. Isoflurane (2-3%) mixed with oxygen enriched air was used for inducing and maintaining the animals under anesthesia. Once anesthetized, 2 of the animals had a unilateral transtympanic injection of 150uL liquid Poloxamer solution in their left middle ear and 50uL in their left external ear. The solution immediately jellified and the animals were then placed on to the MRI support. After acquiring axial and coronal T2 Turbo RARE anatomical reference scans (TR/TE=2500/33ms, RARE factor=8), B0 was shimmed over the brain using the Mapshim procedure. Axial gradient-echo EPI images with the same geometry as the anatomical reference (TR/TE=1000/18ms, FA= 54deg, resolution=0.364x0.364x0.7mm) were acquired to assess the effect of the gel on susceptibility artifacts. Following the EPI sequence, animals were taken out of the scanner and their contralateral ear canal was filled following the same procedure as described above. After the experiment, the animals were placed back in their cage and monitored every day during one week. Their behavior was compared to the two non-injected animals present in the same cage.Results and Discussions
All animals tolerated and recovered well from the procedure and did not show any signs of deficits in social behavior, self-mutilation or weight loss. Furthermore, rats kept reacting to loud sound, demonstrating that they were not completely deaf. The benefits of substituting the air in the middle and outer ear with a water-based solution can be appreciated on Fig.1-2. The representation of the ventral left side of the brain was entirely recovered, whereas on the right side strong susceptibility artefacts can be observed. This method gives now access to specific BOLD signal variation in key neuro-psychiatric areas such as the amygdala and hypothalamus. In addition, this on-going study aims at evaluating the long terms effect of Poloxamer in the ear system and its suitability for longitudinal fMRI experiments. It has been reported in Guinea pigs that Poloxamer remains stable up to 49 days in the ear with no inflammatory or immunological responses6, which minimizes the number of injections required for longitudinal studies.Conclusion
The present results demonstrate the practical advantages of a fast procedure to minimize susceptibility related signal loss in BOLD fMRI by administering a thermo-reversible Poloxamer gel into the cavities of the rat’s middle and outer ear. In combination with techniques for correcting intervoxel dephasing such as PSF mapping7 it should allow high fidelity neuroimaging to be performed with true whole brain coverage. In addition to making atlas registration more reliable, this method provides the prerequisite for assessing neurofunction of deep-lying structures of the rodent’s brain, particularly by tools such as fMRI combined with optogenetics.Acknowledgements
No acknowledgement found.References
1. Mandeville JB, Marota JJ, Kosofsky BE et al. Dynamic functional imaging of relative cerebral blood volume during rat farepaw stimulation, Magn Reson Med. 1998. Apr;39(4):615–24
2. Li R, Liu X, Sidabras JW et al. Restoring susceptibility induced MRI signal loss in rat brain at 9.4T: a step towards whole brain functional connectivity imaging, PLoS One. 2015; 10(4): e0119450.
3. Singh-Joy SD, McLain VC, Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics, Int J Toxicol. 2008;27 Suppl 2:93-128
4. Devi DR, Sandhya P, Vedha Hari BN, Poloxamer: A Novel Functional Molecule For Drug Delivery And Gene Therapy, J. Pharma Sci & Res, Vol.5(8), 2013, 159 - 165
5. Dumortier G, Grossiord JL, Agnely F et al. A review of poloxamer 407 pharmaceutical and pharmacological characteristics, Pharm Res. 2006 Dec;23(12):2709-28
6. Feng H, Sun J, Jiang P, In vitro and in vivo biodegradation of sustained-release vehicle poloxamer 407 in situ gel, Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008 Jan;22(1):28-31
7. Paul D, Maxim Zaitsev M, Harsan L et al. Implementation and Application of PSF-Based EPI Distortion Correction to High Field Animal Imaging, Int J Biomed Imaging. 2009; 2009: 946271
Figures
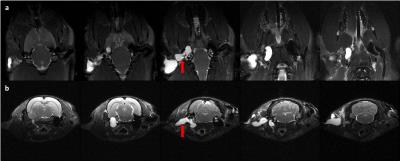
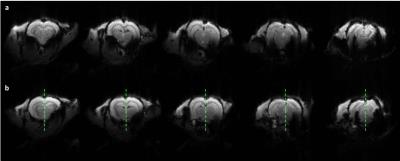
Mean GRE EPI (TR/TE=1000/18ms) images pre (a) and post (b) injection of 20% Poloxamer solution in the same animal, demonstrating its effect on recovering signal losses due to magnetic field inhomogeneities in the lower parts of the brain. The green vertical line in (b) separates the right (non-injected) and left (injected) side of the rat’s brain. One can clearly observe the recovery of deep structure in the left hemisphere.
Note that the animals were also scanned once at baseline, before Poloxamer administration, following the exact same imaging protocol as described in the material and methods section.