5246
Prospective Motion Correction of Multi-Band Multi-Echo fMRI During Overt Speech to Localize the Language Network1Radiology and Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Cognitive Neuroscience, Maastricht University, 3Neurosurgery, St. Lukes Roosevelt Hospital Center, New York, NY, 4Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 5University Hospital Freiburg, Freiburg, Germany
Synopsis
The functional localization of language and other clinically relevant brain networks at the subject-level has been limited by fMRI artifacts such as head motion. These artifacts have forced task-based fMRI for functional localization to be designed around strict timing and strong constraints, such as silent reading in 20-second task-rest blocks. Such limits make experiments hard to follow by patients and not ecologically valid. Dealing with these artifacts, especially at
Introduction
The localization of language networks in the human brain using functional MRI is a key clinical application in the context of neurosurgical planning. However, currently, language localization tasks are limited to non-naturalistic paradigms (20s covert speech, 20s rest, etc), high artifact susceptibility, and low spatial resolution. This is due to the impact of high levels of non-BOLD artifact in data and aggressive preprocessing to deal with this artifact. Here we combine high-fidelity multi-echo multi-band functional MRI with prospective motion correction during a variably naturalistically timed overt speech experiments to localize activation of lateralized language and other language-related networks.Methods
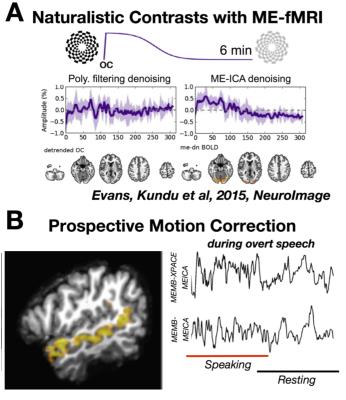
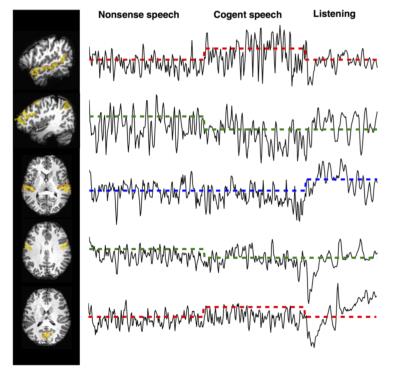
Subjects: Two healthy right handled males (student volunteers) were imaged for this experiment. This study was approved by the institutional IRBs were the experiments were performed. Stimuli: Three stimulus designs were assessed. (A) In a single block-step design of covert reading vs. non-sense speech generation. (B) In an alternating block experiment, the subject was instructed for paced reading from “The Rainbow Passage,” in the sequence (R-rest with crosshair, C-covert reading, O-overt reading): 50s R, 50s C, 50s O, 50s R, 50s O, 50s C, 50s R. (C) In a complex language production-comprehension experiment, three conditions were tested: overt speech of scrambled language, overt speech of cogent language, and passive listening of cogent language. All language corresponded to the same scene from the film “The Good, The Bad, The Ugly.” Each block was 5 minutes long. Imaging: All participants were scanned on a Siemens Magnetom 7T MRI scanner (Siemens, Erlangen, Germany) with a birdcage-transmit/32-channel-receive head coil. Scans with prospective motion correction were acquired based on the Kineticor motion tracking system and the XPACE component addition to a multi-echo multi-band EPI pulse sequence (Zaitsev, 2006, Olafson, 2014). Anatomical imaging included MP2RAGE (0.8mm iso.,TR=6s, TE=5ms, TI=1050ms;3000ms), and 8 minutes of MEMB-fMRI (2.5mm iso.; TR=1.0s, TEs=8.6,20,31,43ms; MB=3; FOV shift ½ GRAPPA=3)6-7. Imaging Processing: MP2RAGE “UNI” images homogenized T1 contrast and attenuated intensity non-uniformity, and were used for brain extraction and masking. MEMB-fMRI analysis involved multi-echo independent components analysis (ME-ICA) with AFNI meica.py with default settings, which implemented: slice time and motion correction; co-registration to masked T1; T2* mapping and weighted combination of echoes; dimensionality estimation with multi-echo PCA (ΔT2* fitting of PC amplitude vs. TE); spatial FastICA decomposition; BOLD/non-BOLD IC selection (ΔT2* fitting of IC amplitude vs. TE), and time series denoising by non-BOLD component removal. Multi-Network Activation Analysis: After ME-ICA identified BOLD components per subject, the separate time course models corresponding to the timing of experimental conditions (e.g. overt speech, covert speech, cogent reading, scrambled reading) were fit to each component time course, with a multiple general linear modeling approach. These models were not explicitly reflecting a filtering to any low (e.g. 0.01 Hz) or high frequency range, which was also true of the data due to the processing by ME-ICA.Results
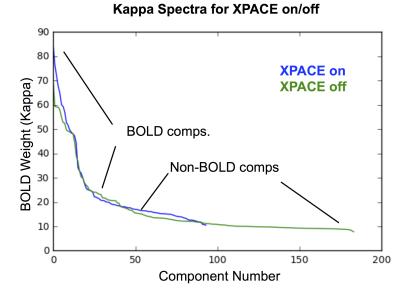
ME-ICA on language-network activation data acquired with MEMB-XPACE and non-XPACE EPI sequences showed a comparable space of BOLD components based on ME-ICA Kappa spectra (Figure 1). From experiments to activate the language network with naturalistic stimulus timing (1-5 min blocks), single components at the subject level functionally localized superior temporal gyrus leading to Wernicke’s area and inferior frontal gyrus proximal to Broca’s area. The ability to capture activity on variable and naturalistic time scales was due to the removal of non-BOLD drifts and due to the isolation of BOLD components. The BOLD components generated by ME-ICA of these data were also compatible with the multi-network activation mapping strategy. The time course models of activation reflecting naturalistically timed long-block overt speech experiments were fit to the component time courses. Comparing component time courses with a clear language-related activation showed, for the non-XPACE sequence, a smaller mean activation magnitude versus the activation of the XPACE sequence. This was associated to increased spin-history artifact due to high head motion incurred without the use of XPACE (Figure 2). A similar observation was made for the alternating covert/overt reading experiment (Figure 3). The language network was also seen related to a time course correlated to the stimulus block with overt recitation of cogent language, but not scrambled language.Discussion
These approaches and results, which are readily translatable to 3T MRI, could prove instrumental in improving neurosurgical localization in children, where functional variable neuroanatomy and unpredictable functional lateralization of language networks and other functions is observed.Acknowledgements
No acknowledgement found.References
Zaitsev M., Dold C., Sakas G., Hennig J., Speck O. (2006). Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. Neuroimage 31, 1038–1050. 10.1016/j.neuroimage.2006.01.039 Herbst M., Maclaren J., Lovell-Smith C.,
Sostheim R., Egger K., Harloff A., et al. . (2014).Reproduction of motion artifacts for performance analysis of prospective motion correction in MRI. Magn. Reson. Med. 71, 182–190. 10.1002/mrm.24645
Olafsson, V., Kundu, P., Wong, E. C., Bandettini, P. A., & Liu, T. T. (2015). Enhanced identification of BOLD-like components with multi-echo simultaneous multi-slice (MESMS) fMRI and multi-echo ICA. Neuroimage, 112, 43-51.
Marques, José P., et al. "MP2RAGE, a self bias-field corrected sequence for improved segmentation and T 1-mapping at high field." Neuroimage 49.2 (2010): 1271-1281.
Figures