5242
The clinical relevance of correcting susceptibility-related distortions in presurgical fMRI at 7 T1High Field Magnetic Resonance Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Study Group Clinical fMRI, Department of Neurology, Medical University of Vienna, Vienna, Austria
Synopsis
The clinical relevance of correcting susceptibility-related distortions using a recently-developed dynamic distortion correction (DDC) approach is assessed. This was applied in fMRI data acquired from a group of 12 patients with a range of neuropathologies at 7 T. Despite the presence of pathologies, time series of artifact-free field maps were generated. If distortion correction was neglected, substantial displacements, both in EPI geometry and activation, were observed. Two cases with potential clinical implications were identified. The DDC was able to accurately correct distortions in all cases and is shown to be effective and clinically relevant in presurgical planning at 7 T.
Purpose/Introduction
In presurgical planning, susceptibility-related
distortion of fMRI results can lead to clinically relevant mislocalization of
activation and affect decisions concerning optimal therapeutic approach.1 Acquisition at UHF (7 T) with the
associated increase in time-series SNR and BOLD sensitivity2-4 allows higher resolution and/or
shorter measurement time, which is valuable in preoperative planning. However,
field inhomogeneities increase with B0, increasing image distortion.
We assess distortion-related shifts in activation and their potential clinical
relevance in twelve patients with a range of neuropathologies and the
effectiveness of a novel method for Dynamic Distortion Correction (DDC)5 in removing distortions.Subjects and Methods
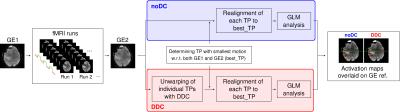
Twelve patients with brain tumors and arteriovenous malformations volunteered to participate in this study, which simulates presurgical fMRI localization of the primary motor cortex (M1) at 7 T. One patient was excluded due to excessive motion. Ten runs (of 56 volumes each) of a motor task were presented in block design. EPI time-series were acquired with 1.7×1.7×3.0 mm3 voxels, matrix size 128×128×40, TE/TR = 22/2500 ms, GRAPPA 2, RBW = 1447 Hz/px. Additionally, multi-echo GE data (TE = [5.0,10.0,16.0] ms) were acquired before and after the fMRI runs. Data were preprocessed and analyzed with SPM12. Analysis pipelines are illustrated in Figure 1. The analysis for no distortion correction (noDC) and DDC differs only in the unwarping of individual time points (TPs). DDC uses the GE pre-scan to identify the contribution to the phase that does not originate from B0. This contribution is subtracted from the phase of each single-echo EPI time point to generate a series of field maps from the fMRI data itself. These are converted into voxel shift maps (VSMs), which are then used to bring the fMRI runs into a distortion-free space.Results
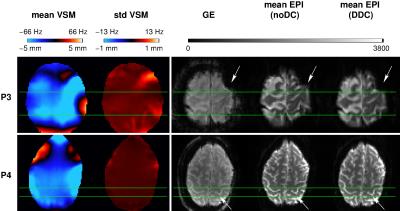
Comparison of DDC and noDC EPI runs with reference GE images indicated that warps seen in noDC data were effectively removed by DDC in all patients. Figure 2 illustrates two cases in which susceptibility-related geometric distortion resulted in clinically relevant shifts in activation. In P3, a large region of susceptibility-related distortion was visible in M1 in the mean noDC EPI. Here, voxel shifts up to 5.1 mm (see “mean VSM”) were found. The std VSM showed relatively larger values (~1 mm) in the vicinity of the pathology for this patient. In P4, a shift in M1 of ~3.4 mm and low std VSM were observed. These shifts were corrected accurately by DDC as demonstrated by the good correspondence to the distortion-free GE reference. Smaller shifts of ~2 mm were present in four patients and to a negligible extent in the remaining four.
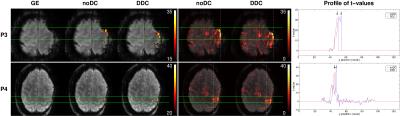
Shifts in activation of ~3.4 mm were observed in three patients, reaching over 5.1 mm in more lateral areas of M1 in P3 (Figure 3). These shifts were smaller (~1.7 mm) in five patients, and negligible in two. Two cases with clinically relevant shifts in activation were found (P3 and P4, Figure 3). In P3, activation may wrongly be judged to originate from the strong susceptibility-related artifact. With DDC, however, the activation perfectly overlays M1. In P4, with noDC, the most statistically significant activation appeared to be in the middle part of the precentral gyrus, hindering unique identification of the central sulcus. The activation could be interpreted as reflecting viable primary motor cortex. Following DDC, activation was shifted into the post-central gyrus, a correction which was established as being accurate (see P4, Figure 2). In this case, DDC is likely to change the clinician’s interpretation to suggest that, in fact, primary motor cortex is compromised and function has been reallocated to the post-central gyrus.
Discussion
A dynamic approach to correcting for distortions was used in this study, as “static” approaches do not account for changes in B0 originating from unintentional or task-related motion,7 respiration8-10 or hardware heating.11 The higher standard deviation values observed in VSMs close to pathologies reveal the dynamic nature of distortion in these regions. Measurable activation displacements of 1.7 up to 5.1 mm (1-3 voxels) were observed in M1. These dislocations were accurately corrected with the DDC approach. The shifts observed in both EPI geometry and functional results are consistent with prior investigations involving neurological patients at 7 T, which report distortions of ~4 mm or even larger, pointing to a possible misidentification of the central sulcus.1,12Conclusion
If neglected, EPI distortions may lead to misinterpretation and/or mislocalization of activation, particularly in presurgical fMRI, where significantly altered morphology and contrast are present due to pathology. Hence, we propose fMRI data to be unwarped into a distortion-free space using a dynamic approach before drawing conclusions for presurgical planning.Acknowledgements
This study was funded by the Austrian Science Fund (KLI264). BD was additionally supported by a DOC fellowship of the Austrian Academy of Sciences.References
1. Dymerska B, Fischmeister F, Geissler A, et al. Clinical Relevance of EPI distortion Correction in Presurgical fMRI at 7 Tesla. Proceedings of the Twenty-third Annual Meeting of the ISMRM, Milan 2014; #1416.
2. Beisteiner R, Robinson S, Wurnig M, et al. Clinical fMRI: Evidence for a 7T benefit over 3T. Neuroimage 2011; 57(3):1015-21.
3. Triantafyllou C, Hoge RD, Krueger G, et al. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. NeuroImage 2005; 26(1):243-50.
4. van der Zwaag W, Francis S, Head K, et al. fMRI at 1.5, 3 and 7 T: characterising BOLD signal changes. Neuroimage 2009; 47(4):1425-34.
5. Dymerska B, Poser BA, Barth M, et al. A method for the dynamic correction of B0-related distortions in single-echo EPI at 7T. NeuroImage 2016; doi: 10.1016/j.neuroimage.2016.07.009.
6. Andersson JL, Hutton C, Ashburner J, et al. Modeling geometric deformations in EPI time series. Neuroimage 2001; 13(5):903-19.
7. Jezzard P, Clare S. Sources of Distortions in Functional MRI Data. Human Brain Mapping 1999; 8:80-85.
8. Dymerska B, Poser BA, Bogner W, et al. Correcting dynamic distortions in 7T echo planar imaging using a jittered echo time sequence. Magn Reson Med 2016; 76(5):1388-99.
9. Zahneisen B, Asslander J, LeVan P, et al. Quantification and correction of respiration induced dynamic field map changes in fMRI using 3D single shot techniques. Magn Reson Med 2014; 71(3):1093-102.
10. Zeller M, Kraus P, Muller A, et al. Respiration impacts phase difference-based field maps in echo planar imaging. Magn Reson Med 2014; 72(2):446-51.
11. Foerster BU, Tomasi D, Caparelli EC. Magnetic field shift due to mechanical vibration in functional magnetic resonance imaging. Magnetic Resonance in Medicine 2005; 54(5):1261-67.
12. Robinson S, Geissler A, Trattnig S, et al. Correcting for EPI distortion at very high field using the fieldmap method with multi-channel coils: effectiveness in presurgical planning fMRI at 7 T. Proceedings of the Eighteenth Annual Meeting of the ISMRM 2010; #2302.
Figures