5239
Improved functional connectivity between the ventromedial prefrontal cortex and amygdala with multi-echo EPI: a resting state analysis1Applications & Workflow, GE Healthcare, Orsay, France, 2Neuroimaging Unit, Max Planck Institute of Psychiatry, Munich, Germany
Synopsis
EPI suffers from signal loss in the ventromedial prefrontal cortex (vmPFC), a region of special interest in affective neuroscience. Last year, we showed that Multi-echo EPI (MEPI) was performing better than EPI in the vmPFC using a fear conditioning task. In the present work, we used a seed in the vmPFC, derived from the fear conditioning data, for a seed-based analysis on resting-state data collected from the same subjects. We demonstrate that the additional vmPFC cluster extent detected in the fear conditioning task reflects anatomically/functionally relevant activation as it is connected to bilateral amygdala and to the default mode network.
Introduction
A limitation of fMRI in the investigation of affective symptomatology is signal and BOLD sensitivity loss in EPI1, which is particularly pronounced in ventromedial prefrontal cortex (vmPFC). Ironically, this is a region of special interest in affective neuroscience and psychiatry2 given its role in emotion regulation. Multi-echo EPI (MEPI) is known to have several advantages3-9 over EPI. Last year, we have demonstrated using a fear conditioning task10 and the weighted sum combination approach5 on 32 subjects that MEPI showed increased sensitivity in the vmPFC compared to standard EPI11-12.Purpose
We would like to demonstrate that the additional cluster extent identified in the most ventral part of the vmPFC11 reflects relevant activation (instead of non-neuronal noise). We hypothesized that the vmPFC cluster is connected to the bilateral amygdala and the default mode network (DMN) in a separate data-set of resting-state data, in line with previous anatomical and PET-imaging work.Methods
Thirty-two subjects were scanned (age 25.0±3.2 years, 17 female) on a GE Discovery MR750 3.0T scanner with a 32-channel coil.
For each subject, a 3D-T1 (~1mm) was first acquired. Then a 10 minutes resting-state acquisition followed by the fear conditioning task were performed using MEPI. MEPI was acquired with 3 echoes (called E1, E2, E3). Acquisition parameters: 64x64 matrix, 3.4mm resolution, 36 slices, acceleration-factor 2, TR=2.56s, TE1/TE2/TE3=12/29/46ms.
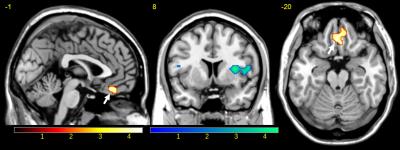
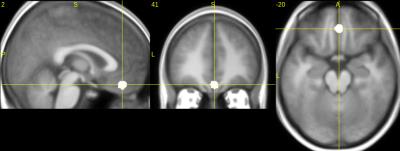
Last year, we used a weighted sum combination approach5 (resulting in the echo-combined called Ec) and compared the contrast “safety stimulus > conditioned stimuli” obtained with E2 (the gold standard with TE2=29ms) and with Ec in the fear conditioning task. The paired t-test Ec>E2 revealed an additional cluster extent in the most ventral parts of the vmPFC (Figure 1). For the resting state analysis, the seed was derived as a 6-mm sphere centered at the center-of-mass of this vmPFC cluster observed in the paired t-test (Figure 1).
The fMRI analysis was performed with SPM8. Data were corrected for slice timing differences and realigned. Then, the echo-combined Ec was computed5. MEPI data were then co-registered to the 3D-T1 and normalized to the MNI space. Next, the nuisance regressors were added to a general linear model (GLM). Nuisance regressors were motion parameters, their derivatives and CompCor12 regressors. Then the data were variance normalized and the mean time course from the seed was extracted for E1, E2, E3 and Ec. Finally, temporal filtering (bandpass [0.01 0.1] Hz) and spatial smoothing (6x6x6mm3) was applied. Voxel-wise family-wise error correction was used (pFWE-voxelwise<0.05).
Results
The vmPFC seed derived from the fear conditioning data (Figure 1) is located at [2 41-20] in MNI coordinates (Figure 2). The vmPFC seed-based analysis results are given in Figure 3. Only Ec showed significant connectivity between the vmPFC seed and the left and right amygdala (blue arrows on Figure 3). No significant connectivity to amygdala was found for the single echoes E1, E2, E3 at our threshold.
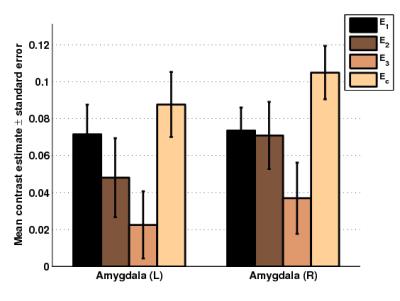
Then, an atlas based (AAL) region-of-interest (ROI) based comparison was performed to examine whether our vmPFC seed was more strongly connected to amygdala in Ec than E2 (see Figure 4). A repeated measures ANOVA showed a significant main effect of “method” (i.e. E1, E2, E3, Ec) for the left amygdala (F(3,93)=3.19,p=0.0272) and the right amygdala (F(3,93)=4.11,p=0.0087). The paired t-test comparison Ec>E2 showed that the contrast estimates of Ec were significantly stronger than the current gold standard E2 (p=0.0303 left amygdala, p=0.0356 right amygdala).
Discussion
Our results show that the additional cluster extent detected in vmPFC in a fear conditioning task reflects anatomically/functionally relevant activation as it is connected to the left and right amygdala. Additionally, when looking at the whole brain pattern on Figure 3, the vmPFC seed analysis revealed typical nodes of the DMN (e.g., posterior cingulate cortex and adjacent precuneus, bilateral inferior parietal lobules). While already partially present in E1 and E2, this pattern was most pronounced in Ec, indicating that the vmPFC participates in the DMN. This result is in accordance with earlier PET studies14 showing that also the most ventral part of the vmPFC is part of the DMN, which has not been reported by the large body of fMRI studies (using standard EPI sequences). This is in accordance with previous work2-14 and a strong advantage of MEPI. Recent methodologies like ME-ICA7-8 and multiband acquisition are expected to further improve the sensitivity of MEPI.Conclusion
We have demonstrated that 1) the additional cluster extent identified in the vmPFC in the fear conditioning task11-12 reflects anatomically/functionally relevant activation and 2) that in addition to the mPFC, also the more ventral parts of the mPFC are part of the DMN.Acknowledgements
The authors would like to thank Ines Eidner for her help during data acquisition. VS acknowledges financial support from the Bavarian Academy of Sciences and Humanities.References
1. Deichmann R, Josephs O, Hutton C, et al. Compensation of susceptibility-induced BOLD sensitivity losses in echo-planar fMRI imaging. Neuroimage. 2002;15:120-135.
2. Price and Drevets, Neuropsychopharmacology, 2010;35(1):192-216.
3. Posse S. Multi-echo acquisition. Neuroimage. 2012;62:665-671.
4. Posse S, Wiese S, Gembris D, et al. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magn Reson Med. 1999;42:87-97
5. Poser, B. A.; Versluis, M. J.; Hoogduin, et al. BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: parallel-acquired inhomogeneity-desensitized fMRI. Magn Reson Med. 2006;55:1227-1235.
6. Bhavsar S, Zvyagintsev M, Mathiak K. BOLD sensitivity and SNR characteristics of parallel imaging-accelerated single-shot multi-echo EPI for fMRI. Neuroimage. 2013;84C:65-75
7. Kundu P, Inati S, Evans J, et al. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage. 2012;60:1759-1770. 8. Kundu P, Brenowitz N, Voon V, et al. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc Natl Acad Sci USA. 2013;110:16187-16192
9. Fernandez B, Czisch M. Simulation of BOLD Sensitivity of Single-Shot Multi-Echo EPI versus Sample-Induced Susceptibility Gradients. ISMRM, 2014;2982
10. Fullana M, Harrison B, Soriano-Mas C, et al. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry. 2015; 1476-5578.
11. Fernandez B, Leuchs L, Sämann P, Czisch M, Spoormaker V. Improved detection of fMRI activity in ventromedial prefrontal cortex using multi-echo EPI. ISMRM, 2016 (3722)
12. Fernandez B, Leuchs L, Sämann P, Czisch M, Spoormaker V. Application of multi-echo EPI on a fear conditioning task: evidence of improved BOLD detection in ventromedial prefrontal cortex. Magn Reson Mater Phy, ESMRMB Congress (2016) 29 (Suppl 1):S247–S400 (338)
13. Behzadi, Y., Restom, K., Liau, J. & Liu, T.T., 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 2007;37(1):90-101.
14. Buckner, R.L., Andrews-Hanna, J.R. & Schacter, D.L., 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci, 1124, pp.1-38
Figures