5238
Multi-slice balanced SSFP is an excellent alternative to GE-EPI for rodent fMRI at ultra-high field1Centre d'Imagerie Biomédicale, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Synopsis
Balanced SSFP (bSSFP) can be used as an alternative to gradient-echo (GE)-EPI for BOLD fMRI when image distortions and signal drop-outs are severe at high field. However, on animal systems, 3D-bSSFP acquisitions have low temporal resolution due to limited acceleration options and single slice offers insufficient coverage. Here, we perform multi-slice bSSFP in a pseudo-steady-state and show that non-distorted BOLD fMRI activation maps can be obtained, with comparable performance to GE-EPI. Future work will focus on resting-state fMRI using bSSFP and on the exploration of bSSFP BOLD contrast mechanisms at 14 Tesla.
Purpose
Gradient echo EPI (GE-EPI) is the most widespread sequence for BOLD fMRI due to its high CNR and temporal resolution. However, at ultra-high field (UHF), GE-EPI is corrupted by severe distortions and signal drop-outs, especially in the small-sized brains of rodents.
Balanced SSFP (bSSFP) fMRI has been explored as an alternative, mainly in human1-5 but also in animal studies6-8. However, on animal scanners, 3D acquisitions – particularly well suited for bSSFP to maintain a steady-state – have deterrently low temporal resolution due to currently limited acceleration options (fast read-outs are artefacted and availability of multi-channel coils limited). Though a four-fold acceleration with compressed sensing has been recently demonstrated9, animal studies have so far limited themselves to single slice6-9. Here, we perform multi-slice bSSFP (ms-bSSFP) fMRI in the rat brain at 14T, show that a pseudo-steady-state can be established, and compare the performance of ms-bSSFP and GE-EPI for BOLD fMRI.
Methods
The bSSFP protocol was: 5 1-mm slices, TE/TR=3.0625/6.125ms, matrix: 64x32, 16 dummy scans, in-plane resolution: 300x400μm2. It was designed to match GE-EPI coverage, voxel volume and temporal resolution (TRglobal=1.5s).
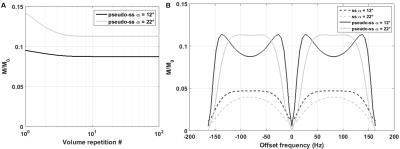
Bloch equation simulations were used to generate the bSSFP signal profile for this protocol as a function of frequency offset both in the conventional and ms-bSSFP steady-state, for various flip angles (α). Relaxation times measured in the rat cortex at 14T were used: T2=25ms, T1=2200ms.
All experiments were approved by the local Service for Veterinary Affairs and performed on a 14T Varian system using a quadrature surface coil. Seven Sprague-Dawley rats underwent an fMRI session under medetomidine sedation10 (0.1mg/kg bolus and 0.1mg/kg/h perfusion).
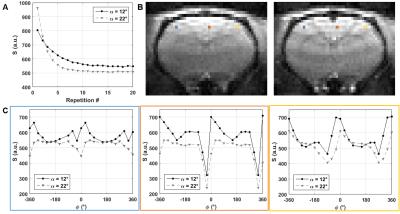
Experimental ms-bSSFP profiles were obtained for α=12°/22° by sampling RF phase-cycling angles φ=0:30:360° in random order.
For task-fMRI, three acquisition protocols were compared: GE-EPI (TE/TR = 17ms/1.5s, α=62°, in-plane resolution: 359x359μm2, 5 1-mm slices) and ms-bSSFP as above with α=12° and 22°, φ=180°. A pair of electrodes was inserted in each forepaw. The stimulation paradigm was 21s OFF - 21s ON (pulse duration: 0.3ms, current: 2mA, frequency: 9Hz) repeated for 5min, with 5min rest between runs. Data was acquired on the same paw for two consecutive runs, with two of the three acquisition protocols (randomly combined), before switching to the other paw, etc. Six to ten runs were acquired on each rat.
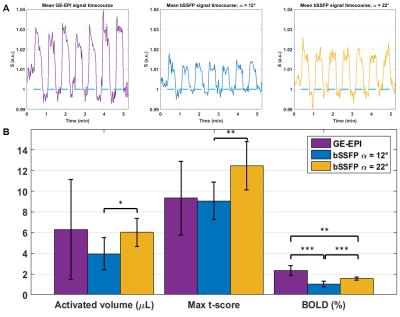
The data were processed in SPM12 using familywise error correction and p<0.05. Maximum t-score, cluster volume and BOLD signal amplitude (averaged over the four voxels with highest activation) were extracted for each cluster.
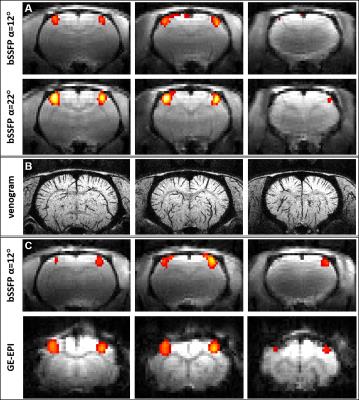
A venogram was also acquired using a 3D-GE sequence (TE/TR=15/30ms, α=20°, matrix: 256x128x128 / 100-μm isotropic resolution)11 for anatomical reference.
Results
A pseudo-steady-state can rapidly be established in ms-bSSFP (Fig.1A) but the optimal α should be increased relative to the steady-state to achieve a plateau for passband bSSFP (Fig.1B). The experimental signal behavior and profiles agreed with simulations (Fig.2).
All protocols detected specific and reproducible activation in the contralateral somatosensory cortex of the rat (Fig.3). The ms-bSSFP with α=22° had – at least – similar activated volume and t-score to GE-EPI, in spite of lower BOLD amplitude (Fig.4). The ms-bSSFP with α=12° had poorer performance in spite of its potential sensitivity to small frequency shifts due to the “bowl-shaped” profile. Overall, fMRI metrics had lower variability with bSSFP than with GE-EPI, and bSSFP images were less distorted.
Discussion and Conclusions
ms-bSSFP can replace 3D-bSSFP when good temporal resolution is required and acceleration options are limited (as for fMRI on animal scanners). A ramped preparation12 can reduce the number of dummy scans to improve the efficiency of ms-bSSFP.
Given the high sensitivity of bSSFP to frequency shifts, even a suboptimal acquisition (ms-bSSFP with α=12°) allows a good detection of activation. With an optimized passband profile (ms-bSSFP with α=22°), bSSFP performance is as good as GE-EPI for BOLD fMRI, with decreased variability and much reduced artefacts. This sequence is therefore an excellent candidate for rodent BOLD fMRI at UHF.
Due
to the improved coverage (compared to single-slice), future work will focus on measuring
resting-state fMRI networks in the rat brain with ms-bSSFP. Volume
coils will be better suited for whole-brain applications than surface coils,
given the dependence of the profile on flip angle. Good shimming (Δf~30-50 Hz) over an extended volume also needs careful consideration.
The contrast mechanisms of bSSFP BOLD fMRI have been studied extensively2-3,5-6,13-14, but never at such high field. BOLD contrast changes with TE, TR and phase-cycling, as well as comparison with simulations, can help determine the weight of contributions from intravascular water, extravascular water around capillaries, or extravascular water around veins, for given sets of parameters.
Acknowledgements
The authors thank Mario Lepore and Stefan Mitrea for technical help with animal setup and monitoring. This work was supported by the Centre d'Imagerie Bio-Médicale (CIBM) of the University of Lausanne (UNIL), the Swiss Federal Institute of Technology Lausanne (EPFL), the University of Geneva (UniGe), the Centre Hospitalier Universitaire Vaudois (CHUV), the Hôpitaux Universitaires de Genève (HUG) and the Leenaards and the Jeantet Foundations.References
1. Scheffler K, Seifritz E, Bilecen D et al., Detection of BOLD changes by means of a frequency-sensitive trueFISP technique: preliminary results. NMR Biomed 2001;14:490-6.
2. Zhong K, Leupold J, Hennig J and Speck O, Systematic investigation of balanced steady-state free precession for functional MRI in the human visual cortex at 3 Tesla. Magn Reson Med 2007;57:67-73.
3. Miller K, Smith SM, Jezzard P et al., Signal and noise characteristics of SSFP FMRI: a comparison with GRE at multiple field strengths. NeuroImage 2007;37:1227-36.
4. Lee JH, Dumoulin SO, Saritas EU et al., Full-brain coverage and high-resolution imaging capabilities of passband b-SSFP fMRI at 3T. Magn Reson Med 2008;49:1099-110.
5. Kim TS, Lee J, Lee JH et al., Analysis of the BOLD characteristics in pass-band bSSFP fMRI. Int J Imag Syst Tech 2012;22:23-32.
6. Park SH, Kim T, Wang P and Kim SG, Sensitivity and specificity of high-resolution balanced steady-state free precession fMRI at high field of 9.4 T. NeuroImage 2011;58:168-76.
7. Muir ER and Duong TQ, Layer-specific functional and anatomical MRI of the retina with passband balanced SSFP. Magn Reson Med 2011;66(5):1416-21.
8. Cheng JS, Gao PP, Zhou IY et al., Resting-state fMRI using passband balanced steady-state free precession. PLoS One 2014;9(3):e91075.
9. Han PK, Park SH, Kim SG and Ye JC, Compressed sensing for fMRI: feasibility study on the acceleration of non-EPI fMRI at 9.4T. Biomed Res Int 2015;2015:131926.
10. Weber R, Ramos-Cabrer P, Wiedermann D et al., A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. NeuroImage 2006:29:1303-10.
11. Park, S.H., Masamoto, K., Hendrich, K. et al., Imaging brain vasculature with BOLD microscopy: MR detection limits determined by in vivo two-photon microscopy. Magn Reson Med 2008;59:855–65.
12. Le Roux P, Simplified model and stabilization of SSFP sequences. J Magn Reson 2003:163(1):23-37.
13. Scheffler K and Hennig J, Is TrueFISP a gradient-echo or a spin-echo sequence? Magn Reson Med 2003;49(2):395-7. 14. Miller KL and Jezzard P, Modeling SSFP functional MRI contrast in the brain. Magn Reson Med 2008;60:661-73.
Figures