5237
Motion-Robust Fetal Brain Imaging using Inner Echo Volumar Imaging1Institute for Systems and Robotics / Department of Bioengineering, Instituto Superior Técnico, Universidade de Lisboa, Lisbon, Portugal, 2Instituto de Biofísica e Engenharia Biomédica, Faculdade de Ciências, Universidade de Lisboa, Lisbon, Portugal, 3Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom
Synopsis
The first fetal functional MRI studies used standard 2D multi-slice Echo Planar Imaging acquisitions, relying on post-processing for addressing motion-related effects. To reduce motion sensitivity, the use of Echo Volumar Imaging (EVI) combined with localized excitation has previously been proposed, but the very heterogeneous maternal environment renders selective excitation extremely challenging. We explored a more robust method combining EVI with inner volume imaging. By obtaining selective excitation from a spin-echo, sensitivity to field inhomogeneity is decreased, and spurious contributions from maternal tissue can be avoided. The method was tested in an adult and demonstrated in fetal imaging in utero.
Purpose
The first attempts to study the functional behaviour of the fetal brain in utero used a 2D multi-slice Echo Planar Imaging (EPI) approach1-3. However this requires correction algorithms that account for movement between individual slices posing several post-processing challenges3. Reducing the repetition time can decrease inter-slice motion, but increases motion-induced slice cross talk.
Echo Volumar Imaging (EVI), an extension of EPI which encodes the whole volume after a single RF excitation4, could provide both increased efficiency and avoid variable saturation effects. Although EVI is very sensitive to B0 field inhomogeneities, the lack of air-tissue interfaces, and the longer T2* values in the fetal brain5,6 make this approach potentially favourable.
The combined use of EVI and selective Pencil-Beam (PB) RF excitation7 has previously been suggested to restrict spatial encoding to the fetal brain but high B0 field inhomogeneities outside the fetal brain and fat within the maternal tissue were problematic for PB selectivity8. Here we explore an alternative and more robust approach based on inner volume imaging with spin echoes9.
Methods
The proposed method was firstly tested on healthy adult brain (male, 21 years old) and then in a fetus (gestational age: 29weeks + 3) in utero. The fetus was initially thought to be healthy, but the scan revealed ventriculomegaly. Informed consent was obtained for all examinations.
Scans were performed on a Philips Achieva 3T scanner with 32 channel receiver coils –a cardiac torso coil for fetal imaging and a head coil to image the ventricles in the adult brain.
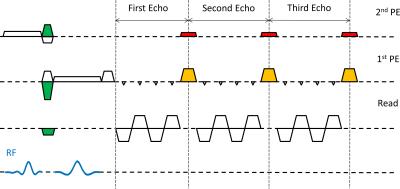
A spin-echo EVI sequence was implemented with slice select excitation and refocusing RF pulses (shown in blue in Figure 1) combined with orthogonal gradients to perform inner volume imaging. A minimum echo time of 24 ms (adult) and 41 ms (fetus) was used to ensure T2* weighted data; to reduce the delays, the pre-winder gradients for in-plane encoding were placed between the RF pulses (green-shaded areas), and the spin-echo matched the center of the first k-space plane. The frequency encode axis was aligned with the excited column. The number of phase encode (PE) lines within each plane of the EVI readout was kept even to improve consistency and avoid ghosting along the second PE direction10.
Imaging parameters were adjusted to keep the acoustic noise below 105dB as measured at isocenter. Image-based shimming was used to minimize static field inhomogeneity (B0 estimated from dual-echo gradient-echo sequence with an echo-spacing of 2.3ms, full brain coverage, 5x5x5 mm3 resolution)11, achieving a 2.8 Hz linewidth across the fetal brain (Figure 2).
EVI acquisitions were performed with a field-of-view (FOV) of 200×57×50 mm3 in the adult, and 200×100×100 mm3 in the fetal brain, encoded respectively at 3.5×3.5×2.5 mm3 and 3.5×3.5×5.0 mm3; 14 k-space planes were acquired with a partial Fourier (PF) factor of 0.7, achieving an effective echo time of 68ms (adult) and 124ms (fetus). The readout durations were 199 and 383ms, respectively. Image reconstruction was performed using ReconFrame (Gyrotools, Switzerland), including PF Homodyne reconstruction. In the adult, EVI images were acquired while selecting different inner volumes within the brain to test the sequence performance. The fetal FOV encompassed the whole brain.
Results
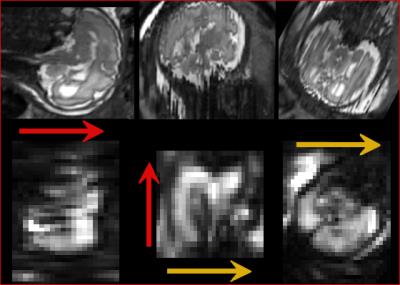
Figure 3 displays the results obtained in the adult brain, demonstrating the ability to selectively image different regions.
Fetal results are shown in Figure 4, where brain anatomy can clearly be seen confirming that the method is feasible. As the fetus moved between the acquisition of the T2-weighted image and the EVI volume, the former was transformed so as to approximately match the position of the fetus during EVI acquisition. The inter-hemispheric fissure is clearly visible in the EVI image.
Discussion
In this study, an EVI readout was paired with inner volume imaging to achieve T2*-weighted images (k-space centre acquired long after the spin echo) with effective outer volume suppression needed to achieve a feasible acquisition matrix. The effective echo times were of the order of the T2* values reported for fetal cortical gray matter at 3.0T6 which is helpful for BOLD sensitivity, but further studies are needed to determine the optimal imaging parameters for fMRI. Increased temporal resolution is achievable with our approach (minimum TR of 430ms for the fetal scan) compared to a standard full-FOV 2D multi-slice method with matching resolution. Decreasing the TR has previously been shown to lead to improvements in physiological noise correction12, but the potential benefits still need to be investigated, particularly the implications of using a spin echo localisation approach.Conclusions
Inner-volume excitation makes fetal EVI feasible without out-of-volume artefacts, paving the way for future fMRI studies.Acknowledgements
Portuguese Foundation for Science and Technology (FCT - IF/00364/2013). ERC funding - dHCP project.References
1. Schopf V, Kasprian G, Brugger PC, et al., Watching the fetal brain at 'rest', Int J Dev Neurosci. 2012;30(1),11–17.
2. Thomason ME, Dassanayake MT, Shen S, et al., Cross-hemispheric functional connectivity in the human fetal brain, Sci Transl Med 2013;5(173),1–10.
3. Ferrazzi G, Kuklisova Murgasova M, Arichi T et al., Resting State fMRI in the moving fetus: a robust framework for motion, bias field and spin history correction, NeuroImage 2014;101(1),555–568
4. Yang Y, Mattay VS, Weinberger DR, et al. Localized echo-volume imaging methods for functional MRI. J Mag Reson Imaging. 1997; 7(2):371-5.
5. Vasylechko S, Malamateniou C, Nunes RG, et al. T2* relaxometry of fetal brain at 1.5 Tesla using a motion tolerant method. Magn Reson Med. 2015; 73(5):1795-802
6. Blazejewska AI, Seshamani S, McKown SK, et al. 3D in utero quantification of T2* relaxation times in human fetal brain tissues for age optimized structural and functional MRI. Magn Reson Med. 2016; In Press, doi:10.1002/mrm.26471
7. Nehrke K, Börnert P, Groen J, et al. On the performance and accuracy of 2D navigator pulses. Magn Reson Imaging. 1999;17(8):1173-81.
8. Nunes RG, Ferrazzi G, Price AN, et al. Combined Echo Volumar Imaging (EVI) and Localized Excitation for Motion Insensitive Fetal fMRI. ISMRM. 2015; 3914.
9. Feinberg DA, Hoenninger JC, Crooks LE, et al. Inner volume MR imaging: technical concepts and their application. Radiology. 1985;156(3):743-7.
10. van der Zwaag W, Francis S, Bowtell R. Improved echo volumar imaging (EVI) for functional MRI. Magn Reson Med. 2006; 56(6):1320-7.
11. Gaspar A, Ferrazzi G, Nunes R, et al. Improving Functional Imaging of the Fetal Brain Using Constrained Image-Based Shimming to Suppress Maternal Fat. ISMRM. 2016; 4303.
12. Price AN, Ferrazzi G, Hutter J, et al. An investigation of Multi-Band imaging for rs-fMRI in the fetus. ISMRM Workshop on Simultaneous Multislice Imaging. 2015.
Figures