5234
Mouse somatosensory fMRI at 9.4T using a single-shot Variable Refocusing Flip Angle 3D GRASE1Center for Neuroscience Imaging Research, Institute for Basic Science, Suwon, Korea, Republic of, 2Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences and Technology, Sungkyunkwan University, Suwon, Korea, Republic of, 3Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of
Synopsis
A single-shot variable flip angle (VFA) 3D GRASE imaging protocol was proposed for mouse somatosensory spin echo fMRI. Given echo-spacing of 24ms and echo train length of 8, 3D GRASE imaging parameters such as phase-encode schedule in partition direction and variable refocusing flip angles were designed to achieve pseudo-continuous signal modulation and effective TE of 40ms. BOLD fMRI experiments were performed using 9.4T MRI with ketamine anesthetized mice. For eight slice acquisition of electrical forepaw simulation fMRI, the proposed single-shot VFA 3D GRASE protocol achieved higher tSNR, t-values and larger activation areas than conventional 2D SE-EPI.
Purpose
Animal fMRI tends to perform on high magnetic fields, which increase the sensitivity to susceptibility effects such as BOLD signals. To reduce distortions of EPI images and to precisely localize activation sites, spin-echo BOLD fMRI has been suggested at high magnetic fields. In human fMRI, 3D GRASE fMRI was proposed to achieve higher sensitivity than conventional 2D spin-echo EPI fMRI1. To achieve better SNR and reduce blurring due to signal modulation in slice (partition) direction in 3D GRASE, flip angles and ordering schemes for phase encoding in slice direction should be carefully designed2-4. In this work, a single-shot Variable refocusing Flip Angle (VFA) 3D GRASE imaging protocol was designed and applied for mouse somatosensory 9.4-T fMRI.Methods
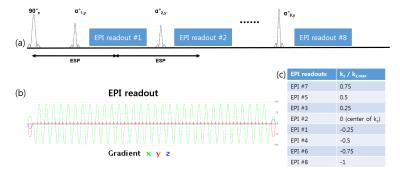
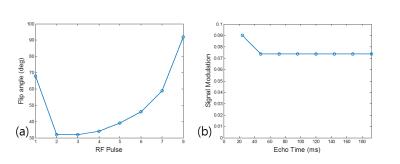
Design of 3D GRASE Protocols: A single-shot VFA 3D GRASE was designed as shown in Fig. 1. The T1 and T2 relaxation times of the target tissue were set to 2000ms and 40ms. With TR of 1500ms, echo train length of 8, and echo-spacing of 24ms due to long EPI readouts, 3D GRASE imaging parameters (phase-encode schedule in partition direction and variable refocusing flip angles) were designed based on extended phase graph theory5 to achieve the effective TE of 40ms. The phase-encode in partition direction was centrically ordered with the center located in the second spin-echo at TE = 48ms. To reduce blurring, target signal modulation was pseudo-continuous, where the signal modulation from the second to the last echo was constant. Refocusing flip angles were designed as shown in Fig. 2(a), leading a constant simulated signal modulation shown in Fig. 2(b). In the reconstruction, the data acquired at the first spin echo were scaled by a constant of 0.82 to make the simulated signal modulation to be constant.
Experiments: The experiments were performed in male C57Bl6/J adult mice (23-30g) with approval of a local Institutional Animal Care and Use Committee (IACUC). Mice were initially anesthetized with a mixture of ketamine and xylazine (100mg/kg and 10mg/kg IP). From 45 minutes after the bolus injection, ketamine (30mg/kg/h) with supplemental fluid of 5% dextrose in saline was administered IP. Animal’s rectal temperature was maintained at 35.5~36.5℃. For electrical forepaw stimulation, a needle (30G) electrode was placed in the right forepaw between digits 2 and 4. Electrical pulse stimulation with 0.5 ms width, 0.5mA current, and 4Hz frequency was given with a constant current stimulation isolator triggered by a pulse generator. BOLD fMRI was acquired using a 9.4T Bruker scanner with a transmit volume coil and a planar receive coil (ID=10mm). Eight contiguous axial slices with slice-thickness of 0.5mm were selected to cover somatosensory cortex. In-plane resolution was 188μm×188µm with FOV of 18mm×12mm and imaging matrix of 96×64. Interleaved acquisitions of a single-shot 3D GRASE and conventional 2D spin-echo EPI, as a comparison, were measured with effective TE = 40ms and TR = 1.5s. For one mouse, conventional 2D multi-slice gradient-echo EPI was acquired with TE = 16ms. Stimulation paradigm was 42s baseline – 21s stimulus – 42s baseline – 21s stimulus – 42s baseline. AFNI software was used for fMRI analysis. Temporal signal to noise ratio (tSNR) were evaluated over images of the first baseline period. BOLD percent changes over a region of interest (ROI) in the contralateral forepaw somatosensory cortex were also compared.
Results
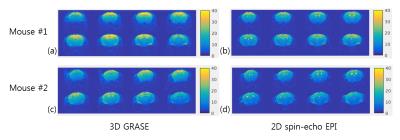
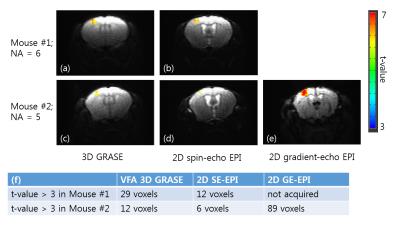
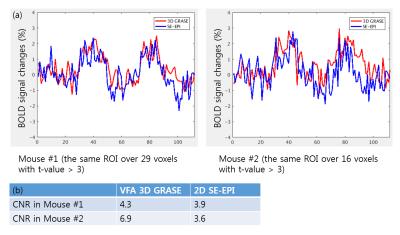
Fig. 3 shows tSNR maps for 3D GRASE and 2D SE-EPI. High-quality GRASE and SE EPI images were obtained. Due to the optimization of GRASE parameters for cortical tissue, the signal intensity of CSF was greatly reduced in 3D GRASE compared to SE-EPI. For most voxels except CSF, tSNR of 3D GRASE is higher than that of 2D SE-EPI. As shown in Fig. 4, BOLD activation area was larger and t-values was higher in 3D GRASE than 2D SE-EPI . As expected, much larger activation area was observed in 2D GE-EPI. Fig. 5 shows BOLD time courses over the same ROI in the contralateral forepaw somatosensory cortex (t-value > 3 in 3D GRASE fMRI). The peak BOLD changes were similar but CNR was higher in 3D GRASE than 2D SE-EPI.Discussion & Conclusion
VFA 3D GRASE protocol was designed to have an effective TE of 40ms given echo-spacing of 24ms and echo train length of 8. For eight slice acquisition of electrical forepaw simulation fMRI, the proposed single-shot VFA 3D GRASE protocol achieved higher tSNR, t-values and larger activation areas than conventional 2D SE-EPI. Our data indicates that VFA 3D GRASE is an attractive fMRI method of animals at high magnetic fields.Acknowledgements
This work was supported by IBS-R015-D1.References
1. Kemper V et al. Sub-millimeter T2 weighted fMRI at 7T Comparison of 3D-GRASE and 2D SE-EPI. Front. Neurosci. 2015; 9:163.
2. Kemper V et al. Variable Flip Angle 3D-GRASE for High Resolution fMRI at 7 Tesla. Magn Reson Med 2016; 76:897-904.
3. Busse R et al. Fast Spin Echo Sequences With Very Long Echo Trains: Design of Variable Refocusing Flip Angle Schedules and Generation of T2 Contrast. Magn Reson Med 2006; 55:1030-1037.
4. Kim et al. Variable-Flip-Angle Single-Slab 3D GRASE Imaging with Phase-Independent Image Reconstruction. Magn Reson Med 2015; 73:1041-1052.
5. Hennig J. Multiecho Imaging Sequences with Low Refocusing Flip Angles. J Magn Reson 1988; 78:397-407.
Figures