5233
Susceptibility Artifact Correction for DBS-fMRI using a PSF Mapping-based Reversed Gradient Approach1Departments of Neurologic Surgery, Mayo Clinic, Rochester, MN, United States, 2Departments of Radiology, Mayo Clinic, Rochester, MN, United States, 3Departments of Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, United States, 4Department of Biomedical Magnetic Resonance, Institute for Experimental Physics, Otto-von-Guericke University Magdeburg, Magdeburg, Germany, 5German Centre for Neurodegenerative Diseases (DZNE), Magdeburg, Germany, 6Leibniz Institute for Neurobiology, Magdeburg, Germany, 7Center for Behavioral Brain Sciences, Magdeburg, Germany
Synopsis
Deep brain stimulation (DBS) fMRI has been considered as an emerging tool in investigating the DBS mechanisms and corresponding clinical outcomes, but suffers from severe susceptibility artifacts near metallic electrodes and tissue/air boundaries. A recent study showed that point spread function (PSF) mapping-based reverse gradient approach has a potential to correct distortions even in gradient-echo echo-planar imaging (GE-EPI) images with opposite phase-encoding polarity using a PSF dataset. To minimize the susceptibility artifacts, in this study, we apply the PSF approach for DBS-fMRI in swine. The results demonstrate that this approach can be beneficial for improving the reliability of DBS-fMRI.
PURPOSE
As deep brain stimulation (DBS) has been widely used for the treatment of many disorders, it is important to understand the underlying mechanisms. fMRI is an emerging tool in investigating the relationship between the DBS mechanisms and clinical outcomes. In general, DBS-fMRI suffers from severe susceptibility artifacts near metallic electrodes for electrical stimulation as well as tissue/air boundaries of the brain, which result in strong intensity and geometric distortions along the phase-encoding (PE) direction in gradient echo echo-planar imaging (GE-EPI). These distortions present a major challenge to perform a faithful data analysis and interpretation. A recent study1 showed that the point spread function (PSF) mapping-based reverse gradient approach can correct distortions in both forward and reverse phase-encoded GE-EPIs with opposite distortions using a single 3D GE-PSF reference dataset acquired with only one PE direction. In this study, we apply the proposed approach for DBS-fMRI in swine to minimize the susceptibility artifacts and investigate the efficiency for improving the reliability of DBS-fMRI studies.METHODS
Eleven healthy pigs underwent unilateral nucleus accumbens DBS-fMRI on a 3T Signa Excite MRI scanner (16.0M4, GE Medical Systems, Milwaukee, WI) using a homemade 6-channel receive-only surface coil. The details of the experimental preparation including DBS surgery are described in previous studies2,3. A unilateral DBS lead was externalized and connected to an external pulse generator for electrical stimulations (5V), which was synchronized to the start time of DBS-fMRI scan. A block paradigm consisted of 5 rest (30 sec.) and electrical stimulation (6 sec.) cycles and ended with the rest period. The PE polarity in GE-EPI was altered, which resulted in a pair of GE-EPIs with opposite geometric distortions, and measured in an interleaved order during DBS-fMRI. The imaging protocol was: TR/TE=1500/40 ms, no partial echo, GRAPPA factor=2, 19 slices, readout bandwidth=651Hz/pixel, FOV=160×160, matrix size=96×96, voxel resolution (x,y,z)=1.7×1.7×2.4 mm3. To calculate kernels for distortion correction of the image pair, PSF reference data were acquired in prior scan using identical imaging parameters with a shorter TE (30 ms) to minimize T2* dephasing effects. The PE polarity yielding dominantly stretched distortions around the electrodes was chosen for the PSF scan. Motion correction and voxel smoothing (using 3 mm Gaussian kernel) were performed using SPM24 in different variants of the EPI series including forward and reverse phase-encoded EPIs, with and without distortion correction and the weighted average of the distortion-corrected EPI pair. Finally, BOLD contrasts for DBS stimulation effects were calculated for comparison. In addition, the number of voxels with signal dropouts at the metallic electrode was counted in the distortion-corrected and the combined images.RESULTS AND DISCUSSION
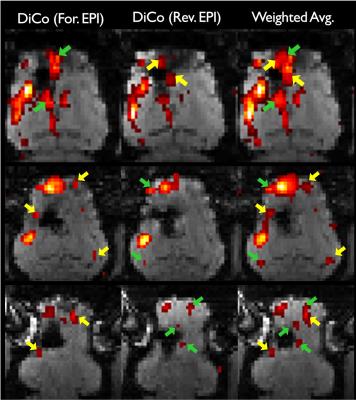
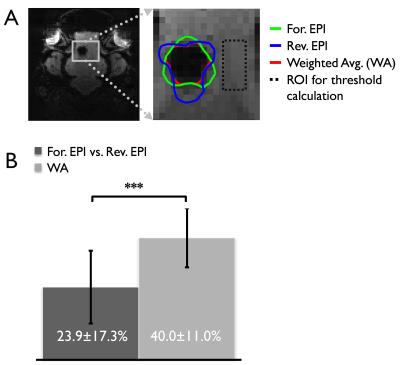
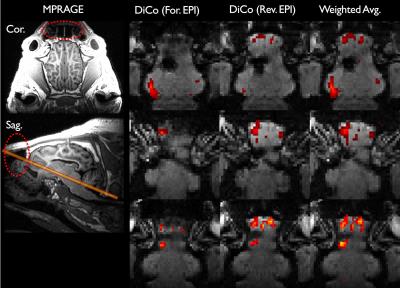
Strongly stretched (yellow arrows) and compressed regions (green arrows) are present in the forward and reverse EPI, respectively due to metal-induced strong field inhomogeneities (Fig. 1). With the proposed distortion correction, the geometric differences between the forward and reverse EPIs were substantially reduced. The anatomy of the distortion-corrected images aligned with each other and the reference image well (see dashed line in Fig. 1), which enabled a reliable combination of the distortion-corrected images. Since severe intra-voxel dephasing effects resulting in strong signal dropouts in GE-EPI, strongly compressed regions manifest in opposite direction in the corresponding GE-EPI pair with opposite PE polarity. We observed some image intensities mismatch even after the distortion correction. Therefore, losses of BOLD contrasts caused by signal dropouts were only partially recovered in the distortions (Fig. 2). Overall a stronger BOLD activation was obtained due to the improvements of the signal-to-noise ratio (SNR) of BOLD sensitivity in the weighted average image series (Fig. 2), but at the cost of temporal resolution reduced by a factor of 2. The number of signal dropout voxels around the metallic electrode (Fig. 3A) was reduced by up to 40% in the combined image (Fig. 3B). Less intensity distortions in either forward or reverse phase-encoded EPIs were observed from 5 and 6 subjects, respectively among the 11 subjects. In addition, this approach resolved the intensity distortions near the tissue-air interfaces (Fig. 4), especially near the frontal lobes, where strong BOLD activation was previously observed3.CONCLUSION
The results demonstrate that the proposed approach could correct geometric distortions of GE-EPI data with reversed PE directions efficiently and could minimize strong susceptibility artifacts round the metallic electrodes as well as in the regions near the tissue/air boundaries in the brain. Therefore, the proposed approach can be a good method to improve BOLD contrast for DBS-fMRI, which is beneficial in investigating the mechanisms of DBS. To improve the temporal resolution of this approach, multi-echo EPI with opposite PE polarity will be considered in future.Acknowledgements
This work was supported by The Grainger Foundation.References
1. In MH, Posnansky O, Beall EB, Lowe MJ, Speck O. Distortion correction in EPI using an extended PSF method with a reversed phase gradient approach. PLoS ONE. 2015;10(2):e0116320. doi: 10.1371/journal.pone.0116320.
2. Min H-K, Hwang S-C, Marsh MP, Kim I, Knight E, Striemer B, et al. Deep brain stimulation induces BOLD activation in motor and non-motor networks: an fMRI comparison study of STN and EN/GPi DBS in large animals. Neuroimage. 2012;63(3):1408-20.
3. Knight EJ, Min H-K, Hwang S-C, Marsh MP, Paek S, Kim I, et al. Nucleus accumbens deep brain stimulation results in insula and prefrontal activation: a large animal FMRI study. PloS one. 2013;8(2):e56640.
4. http://www.fil.ion.ucl.ac.uk/spm/
Figures