5231
High resolution resting-state functional MRI at 7 Tesla using RF parallel transmission1Radiology, University of Minnesota, Minneapolis, MN, United States, 2Center for Imaging of Neurodegenerative Diseases, VA Healthcare System, San Francisco, CA, United States, 3Physikalisch-Technische Bundesanstalt, Berlin, Germany
Synopsis
A major component of the Human Connectome Project (HCP) in the WU-Minn consortium is multiband-accelerated whole-brain resting-state functional MRI (rfMRI) at both 3T and 7T. Although providing better contrast and higher spatial resolutions, the 7T acquisition is compromised by RF nonuniformity. Here, we demonstrate the utility of RF parallel transmission (pTx) for 7T HCP-type rfMRI with 1.6-mm isotropic resolutions. Our results show that pTx can significantly enhance temporal SNR across the entire cortical surfaces and in many subcortical voxels relative to a CP-like-mode RF shimming mimicking single-transmit configurations, thereby holding great potential for acquiring high-quality, high-resolution and high-efficiency rfMRI data.
Purpose
To investigate how RF parallel transmission (pTx) can be used to improve the blood-oxygenation-level-dependent (BOLD) contrast when acquiring Human Connectome Project (HCP)-type whole-brain resting-state functional MRI (fMRI) at 7 Tesla (7T) with 1.6-mm isotropic resolution.Methods
We conducted human studies on a 7T MR scanner (Siemens, Erlangen, Germany), equipped with a prototype pTx system allowing for up to 16 transmit RF channels. Healthy subjects who signed a consent form were scanned using the Nova 8 transmit 32 receive (8Tx/32Rx) head coil (Nova Medical, Inc., MA, USA). The coil was used in the “protected” mode to ensure RF safety. Additionally, band-specific pTx multiband (MB) pulses with single spokes (i.e., RF shimming) were designed using the slab-wise design framework 1. To capitalize on coil geometry and promote RF performance, pulses were designed to image sagittal slices 2. Based on the MB sequence used in the HCP, a new pTx-enabled, 2D gradient-echo echo-planar-imaging (EPI) sequence suitable for BOLD fMRI acquisitions was developed to enable application of multiple pTx MB RF pulses for excitation. We acquired whole-brain resting-state fMRI (rfMRI) data with 1.6-mm isotropic resolutions and 5-fold slice acceleration (MB5) with inverted phase-encode directions (anterior-posterior (AP) and posterior-anterior (PA)) as in the 7T HCP rfMRI protocol 3. In each phase-encode acquisition, a time series of 900 image volumes was acquired, yielding a total of 1800 volumes in total scan time of 33 minutes; this is less than the 1-hour acquisition in the HCP but sufficiently long for proof of principle demonstration. During the data acquisition, the subject was instructed to fixate at a black cross displayed over a white background. For comparison, we also mimicked a single-transmit configuration by performing RF phase shimming targeting a Circularly Polarized (CP)-like-mode 4 RF distribution within the brain, and utilized this shimming to acquire rfMRI data with same imaging parameters as in pTx acquisition. To demonstrate the benefit of pTx, temporal signal-to-noise ratios (tSNR) were calculated after the rfMRI data underwent the HCP spatial 5 and temporal 6 preprocessing pipelines and were compared. As required by the HCP preprocessing pipelines, high-resolution whole-brain T1-weighted and T2-weighted structural images were acquired, both with 0.7-mm isotropic resolution and using the CP-like-mode RF pulses. The T1-weighted image was acquired prior to the rfMRI data acquisition and was also used to derive a brain mask defining the region of interest for the subsequent pTx pulse design. All pulse designs were performed in Matlab (Mathworks, USA).Results
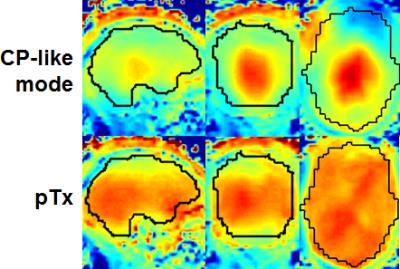
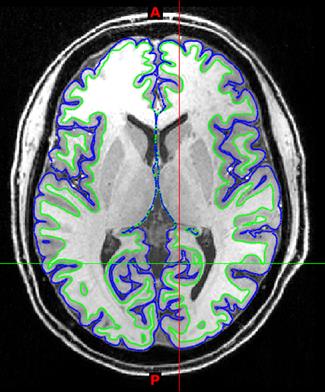
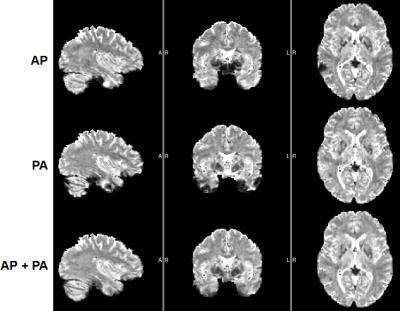
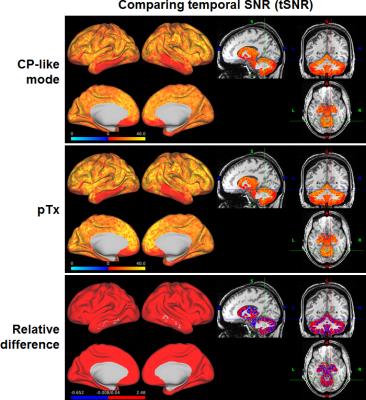
The use of pTx substantially improved RF uniformity across the brain relative to the CP-like-mode counterpart (Fig. 1). Despite of the non-uniform RF distribution across the brain, satisfactory definitions of pial and white surfaces were achieved in most cortical regions when using the CP-like-mode RF shimming for the structural acquisitions (Fig. 2). After undergoing the HCP preprocessing pipelines, the rfMRI data presented little residual distortion or signal dropout (Fig. 3). The improvement in RF uniformity with pTx translated into enhanced BOLD contrast with increased tSNR observed across the entire cortical surface and in many subcortical voxels (Fig. 4). Additionally, the use of pTx resulted in ~36% less RF power delivery (measured as sum of forward minus reflected power across all transmit channels).Discussion
Our results show that pTx can be used to improve the BOLD contrast for 7T HCP-type whole-brain rfMRI with 1.6-mm isotropic resolution while reducing RF power deposition or SAR relative to the CP-like mode RF shimming. The SAR reduction could potentially be traded off for higher slice acceleration (e.g., MB6), to increase data for constant scan time, which is highly desirable for rfMRI data analyses. Our next immediate step is to acquire data for a total scan time of ~1 hour as in the 7T HCP rfMRI protocol and compare pTx data to those obtained with the Nova 1Tx32Rx head coil with dielectric padding as in the HCP. Future work will examine rfMRI with faster volumetric acquisition (i.e. use of higher MB factors), and integrate pTx into structural acquisitions to improve image quality 7,8.Conclusion
We have demonstrated the advantages of pTx over the CP-like-mode RF phase shimming mimicking single-transmit situations when acquiring HCP-type slice-accelerated high-resolution whole-brain rfMRI at 7T. Our data suggest that pTx can be used to enhance BOLD contrast across the entire cortical surfaces and in many subcortical voxels while reducing SAR, thereby providing an attractive means of acquiring high-quality, high-resolution and high-efficiency truly whole-brain rfMRI data that are desired in many neuroscience and clinical applications.Acknowledgements
The authors would like to thank Brian Hanna for setting up computation resources. This work was supported by NIH grants including P41 EB015894 and P30 NS076408.References
1. Wu X, Schmitter S, Auerbach EJ, Ugurbil K, Van de Moortele PF. A generalized slab-wise framework for parallel transmit multiband RF pulse design. Magn Reson Med 2016;75(4):1444-1456.
2. Wu X, Tian J, Schmitter S, Vaughan JT, Ugurbil K, Van de Moortele PF. Distributing coil elements in three dimensions enhances parallel transmission multiband RF performance: A simulation study in the human brain at 7 Tesla. Magn Reson Med 2016;75(6):2464-2472.
3. Vu A, Jamison K, Matthew G, Smith S, Coalson T, Moeller S, Auerbach E, Ugurbil K, Yacoub E. Tradeoffs in pushing the spatial resolution of fMRI for the 7 T Human Connectome Project. NeuroImage In press.
4. Schmitter S, Adriany G, Auerbach E, Ugurbil K, van de Moortele PF. Neither Flat Profile Nor Black Spots: A Simple Method to Achieve Acceptable CP-like Mode Transmit B1 Pattern for Whole Brain Imaging with Transmit Arrays at 7 Tesla. Proc Intl Soc Mag Reson Med 20; 2012; Melbourne, Australia. p 3472.
5. Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 2013;80(0):105-124.
6. Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, Duff E, Feinberg DA, Griffanti L, Harms MP, Kelly M, Laumann T, Miller KL, Moeller S, Petersen S, Power J, Salimi-Khorshidi G, Snyder AZ, Vu AT, Woolrich MW, Xu J, Yacoub E, Ugurbil K, Van Essen DC, Glasser MF. Resting-state fMRI in the Human Connectome Project. NeuroImage 2013;80(0):144-168.
7. Cloos MA, Boulant N, Luong M, Ferrand G, Giacomini E, Hang MF, Wiggins CJ, Le Bihan D, Amadon A. Parallel-transmission-enabled magnetization-prepared rapid gradient-echo T1-weighted imaging of the human brain at 7 T. NeuroImage 2012;62(3):2140-2150.
8. Massire A, Vignaud A, Robert B, Le Bihan D, Boulant N, Amadon A. Parallel-transmission-enabled three-dimensional T2 -weighted imaging of the human brain at 7 Tesla. Magn Reson Med 2015;73(6):2195-2203.
Figures