5223
Quantitative Evaluation of Micro-vessel Blood Flow in Subcortical Nuclei with High-resolution TOF-MRA at 7T1State Key Laboratory of Brain and Cognitive Science, Beijing MR Center for Brain Research, Institute of Biophysics, Chinese Academy of Sciences, Beijing, People's Republic of China, 2University of Chinese Academy of Sciences, Beijing, People's Republic of China, 3Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 4Xuanwu Hospital, Beijing, People's Republic of China, 5Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, People's Republic of China, 6Beijing Institute for Brain Disorders, Beijing, People's Republic of China
Synopsis
TOF-MRA at 7T ultra-high field has been proven to have advantages in imaging the perforating arteries originating from the middle cerebral artery. In this study, a novel method of VOI-based micro-vessel density measurement was developed to quantitatively assess the blood flow in subcortical nuclei. Using this technique, the vascular density (VD) of specific basal ganglia sub-regions can be evaluated. Preliminary results showed that VD values of specific nuclei were different among Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalophy (CADASIL) patients, stroke patients, and healthy volunteers. VOI-based micro-vessel density measurement may be useful in differentiating the etiology of cerebral small vessel diseases.
Introduction
It has been demonstrated that TOF-MRA at 7T can image intracranial perforating arteries originating from the middle cerebral artery (MCA). Previous studies evaluated the perforating arteries by measuring the number, length or tortuosity of its branches, however, these data poorly demonstrate the relationship between vessels and its feeding areas. In our study, a novel method of VOI-based micro-vessel density measurement was proposed to quantitatively assess the blood flow of perforating arteries in specific subcortical nuclei. 7T images of patients with confirmed cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) and stroke have been retrospectively reviewed.Methods
Subjects
Three healthy volunteers and 6 patients were included in this study. Three of the six patients were diagnosed with CADASIL and another three patients were diagnosed with basal ganglion ischemic stroke. The study was approved by the local IRB and written informed consent was obtained from all participants.
Magnetic Resonance Imaging
All participants were scanned using a 7T research system (Siemens, Erlangen, Germany) equipped with a Nova 32-channel head coil. All the patients underwent high-resolution TOF-MRA and T1-weighted (T1w) anatomical imaging. In addition, all participants underwent T1w anatomical imaging on a 3T MAGNETOM Prisma MR scanner (Siemens Healthcare, Erlangen, Germany).
Before the high-resolution TOF-MRA, a TOF-MRA with lower resolution was scanned to provide scout views: voxel size = 0.80x0.80x1.20mm3, time of acquisition (TA) = 1:02min. The high resolution TOF-MRA to visualize the perforators from MCA had the following parameters: FA = 20°, TR = 15ms, TE = 4.30ms, voxel = 0.23x0.23x0.36mm3, FOV = 180x135x46mm3, GRAPPA = 2, TA = 7:34min. T1w anatomical imaging at 7T was scanned with voxel = 0.70mm iso and TA = 9:19min. T1w anatomical imaging at 3T was scanned with voxel = 1.00mm iso and TA =5:53 min.
Data Analysis
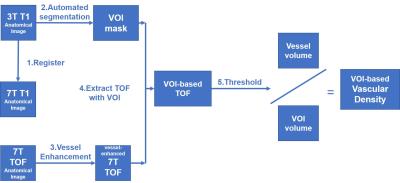
A novel VOI-based method of micro-vessel density measurement was developed in this study. The vessel density was calculated in the regions of subcortical nuclei in individual spaces. The procedure of data analysis is diagramed in Fig.1.
Step 1. Register the 3T and 7T T1w images, and get the VOI masks of subcortical nuclei based on 3T images using FreeSurfer1.
Step 2. Enhance the micro-vessels in TOF-MRA images using VMTK2 toolbox utilizing the Sato algorithm.
Step 3. Mask and threshold the voxels of micro-vessels in specific VOIs by the Otsu algorithm in ImageJ. The vascular density (VD) was calculated as the ratio of the volume of vessels to the volume of the nucleus.
Results
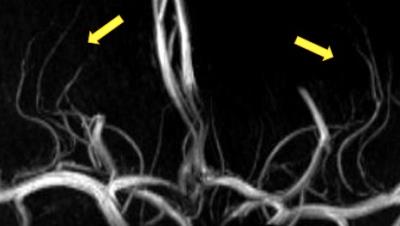
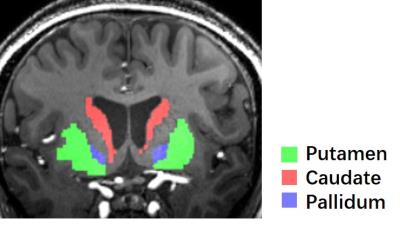
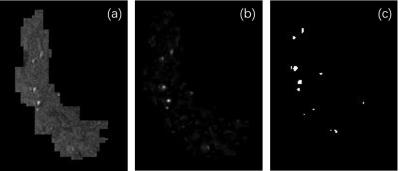
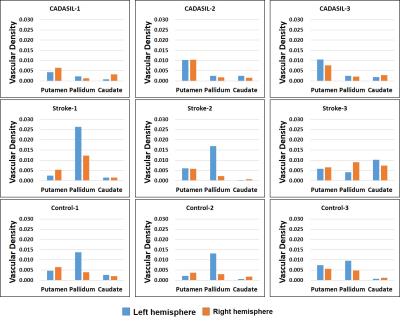
A representative maximal intensity projection of TOF-MRA at 7T was shown in Fig. 2. Visualization of the perforators is qualified for the consecutive processing. The segmented regions of bilateral putamen, caudate, and pallidum was well visualized (Fig. 3). The vessel enhancement suppressed the background tissues and highlighted the flow signal of perforating arteries, providing high-contrast input for binarization (Fig. 4).
The vascular densities of the segmented subcortical nuclei were calculated in all the participants. Their results were listed in Fig. 5. The VD values of the pallidum and putamen CADASIL patients was significantly lower than healthy control (n=6, p=0.013 for pallidum, p=0.023 for putamen).
Discussion
Our preliminary study indicates that the VOI-based VD is a sensitive indicator for evaluating the blood supply to basal ganglia. For CADASIL patients, small vessels were usually impaired. The lower VD of the pallidum and putamen may be due to the lesion of medial lenticulostriate arteries (LSA). On the contrary, in stroke patients, abnormal increase of VD of the left pallidum may due to the collateral vessels.
Different from the previous study3, the vascular density is measured in specific areas, which directly reflects the feeding capacity of perforating arteries to subcortical nuclei. The novel post-processing technique deeply exploits the high-resolution TOF-MRA at 7T MR system. Although studies in larger population are still needed, our novel methodology is promising in understanding the mechanisms of blood flow of perforating arteries in different patient cohort.
Conclusion
The proposed method of VOI-based micro-vessel density measurement is efficient in evaluating blood flow of perforating arteries in CADASIL and stroke patients. Furthermore, VD values may be used as new quantitative tool for monitoring the course of small vessel disease.Acknowledgements
We acknowledge our collaborators for providing patients and their clinical information: Haiqiang Qin from Tiantan Hospital, Xiaojing Fang from Peking University First Hospital. This work was supported in part by Chinese MOST grants (2015CB351701, 2012CB825500), NSFC grant (91132302), and CAS grants (XDB02010001, XDB02050001).References
1. Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., ... & Montillo, A. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341-355.
2. http://www.vmtk.org/index.html
3. Cho, Z. H., Lee, Y. B., Kang, C. K., Yang, J. W., Jung, I. H., Park, C. A., ... & Kim, Y. B. (2013). Microvascular imaging of asymptomatic MCA steno-occlusive patients using ultra-high-field 7T MRI. Journal of neurology, 260(1), 144-150.
Figures