Sankar Seramani1, Lydiane Hirschler2, Boominathan Ramasamy1, Sakthivel Sekar1, Kishore Bhakoo1, Emmanuel Luc Barbier2, and Kuan Jin Lee1
1Laboratory of Molecular Imaging, Singapore Bioimaging Consortium, Singapore, Singapore, 2Université Grenoble Alpes, Grenoble Institut des Neurosciences, Grenoble, France
Synopsis
Arterial
Spin Labeling (ASL) is a non-invasive MRI technique which can be used to
measure quantitative renal perfusion without the injection of contrast agents.
The
goal of this study is to compare the performance of pCASL with FAIR in
measuring Renal Blood Flow in mouse kidney at Ultra
High Field (UHF) MR. Based on our experimental results, pCASL
based perfusion measurement shows similar reproducibility when compared to FAIR
method. pCASL
shows better SNR sensitivity and lower in ROI variation of RBF in the kidney
when compared to FAIR based ASL method at 9.4 Tesla.
Introduction
Gadolinium
based contrast agents are currently used in clinically to determine renal
perfusion, but these contrast agents are contra-indicated for the patients with
end stage renal disease. Arterial Spin Labeling (ASL) is a non-invasive MRI
technique which can be used to measure quantitative renal perfusion without the
injection of contrast agents. The goal of this study is to compare the performance
of pCASL with FAIR in measuring Renal Blood Flow in mouse kidney at Ultra High Field (UHF) MR.Materials and Methods:
MR Imaging experiments carried out in this study
were approved and in compliance with IACUC (Institutional Animal Care and Use
Committee). MR imaging was performed on 9.4T Bruker Biospec scanner/ Avance
III Console/Paravision 6.01 using a 86 mm volume transmit and 4-channel phased
array receive coil. Four male Balb/C mice were used in this study. The animals were
initially anesthetized with 3% isoflurane and subsequently maintained at 2-3%
isoflurane during the scan. Their respiration rates were closely maintained
between 50-60 bpm (~1100ms)
to match the labelling duration (3000ms) and the post labelling delay (300ms).
The labeling ends at 3300ms after 3 respiration cycle (3 x 1100ms),
so that the image acquisition starts at the quiescent
expiration period of the respiratory cycle during the pCASL experiments as
reported earlier by Duhamel et al
[2].MR Experiments:
pCASL sequence parameters were adapted from a previous
report on rat brain [1]. Unbalanced labeling pCASL pulses were applied in the mouse
aorta (at ~1cm away from the iso-center) for 3s followed by a 300ms
post-labeling delay. The labeling pulse train consisted of Hanning window
shaped RF pulses with B1 of 5μT, pulse duration/pulse rate of 400μs/800μs.
Gmax/Gave = 45/5 mT/m, 3D Mapshim procedure was done on the entire abdominal
area covering both the imaging and labelling slice before the imaging experiment. Both label and control phase optimization pre-scans were
performed with a slice thickness= of 4mm,
labeling duration τ= 1.5s,
No of Repetition=1. The optimal labeling phase was computed
and used to measure the actual pCASL perfusion measurement as reported earlier
[1]. For the pCASL measurement, Labelling duration of τ=3s and Post
Labelling Delay (PLD) of 300ms were used. Image acquisition was performed at
the iso-center with single-shot EPI with FOV=3x3cm², slice thickness=1.5mm, matrix=128x128,
TE=22ms, TR=4000ms, 30 repetitions. To quantify RBF, T1 maps were acquired with single shot EPI with slice selective
inversion at 10 TI’s ranging from 30 to
8000 with a TR of 10000) and inversion
efficiencies were measured with fcFLASH sequence
at ~4 mm below the labeling slice with PLD=0 and Labeling duration of 200ms. A
FAIR based PASL dataset was also measured with 11 TI’s ranging from 30 to 10000
and TR=12000. The FAIR-derived CBF and labeling efficiency
were compared with fcFLASH based acquisition. Perfusion
imaging experiments were also carried on a single mouse kidney on different
days to determine the reproducibility of CBF derived using pCASL. Results and Discussion:
In
order to measure the reproducibility of the experiment, we used a single mouse imaged
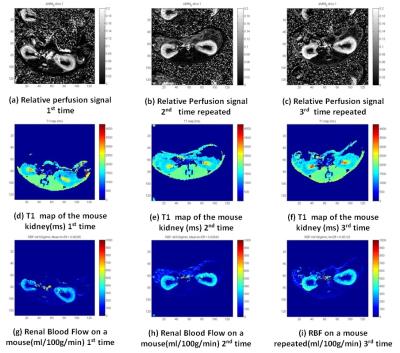
on different sessions over three days. Figure
1(a)-(i) shows the results of pCASL based perfusion imaging on a mouse imaged thrice
to access the reproducibility. The perfusion determined is using
either pCASL or FAIR was comparable,
with a range of 90 and 85 ml/100g/min respectively. The reproducibility of an
ASL experiment is highly dependent on several factors such as motion artefact, respiration
rate, location of the labelling and imaging slices relative to iso-center etc.
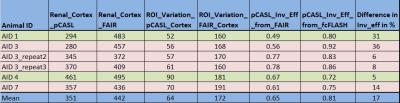
The quantitative results of these measurements and measurements on other mice
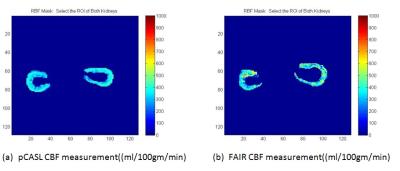
are tabulated in Table 1. Figure 2 shows the comparison of pCASL vs FAIR
measurement on a mouse after masking the background. Based on our results the
RBF values measured with pCASL were 351±64, and 442±172 ml/100gm/min with FAIR.
The inversion efficiency for pCASL measured with fcFLASH and FAIR were around 81%
and 65% respectively, which is comparable to the range of inversion efficiency
derived by FAIR around 61-65% reported by Duhamel et al [2] on the mouse kidney at 11.7 Tesla. Conclusion:
In summary, pCASL based perfusion
measurement shows similar reproducibility when compared to FAIR method. The
difference in labeling efficiency estimated with fcFLASH and FAIR methods is around
17 % for all the experiments. pCASL shows better SNR sensitivity and lower in ROI
variation of RBF in the kidney when compared to FAIR based ASL method. Future
work will concentrate on improving reproducibility of kidney ASL by controlling
the respiration rate at the optimal level, position of imaging and labeling
slice to provide higher labeling efficiency, optimization of shim volumes etc.Acknowledgements
No acknowledgement found.References
[1] Hirschler et al, Proc of ISMRM 2015. [2]Duhamel
et al, Magn Reson Med 2014.