5184
Fat Suppressed Highly Accelerated Dynamic Imaging utilizing View Sharing, Compressed Sensing and Parallel Imaging1Global MR Applications & Workflow, GE Healthcare, Hino, Tokyo, Japan, 2Global MR Applications & Workflow, GE Healthcare, Madison, WI, United States, 3Global MR Applications & Workflow, GE Healthcare, Menlo Park, CA, United States, 4MR Engineering, GE Healthcare, Hino, Tokyo, Japan, 5Global MR Applications & Workflow, GE Healthcare, Houston, TX, United States
Synopsis
DISCO (Differential Subsampling with Cartesian Ordering) is high spatial-temporal imaging technique with Dixon based fat suppression for 3D volumetric Abdominal imaging. We developed DISCO with frequency selective presaturation pulse for fat suppression (FatSAT) called FatSAT DISCO. The feasibility study explores the computational advantage of FatSAT DISCO in accelerating scan time with compressed sensing technique and demonstrated that it is a promising technique for achieving faster imaging for 4D dynamic MR imaging with robustness to image artifact and light computation demand for clinical use.
Purpose
Time resolved T1 weighted 3D sequence has been useful to detect and characterize focal observations and to evaluate arterial vasculature for treatment planning. The current research interest aims at achieving high spatial and temporal resolution for accurate tumor size estimation and reliable lesion detection by temporal information. Recently several methods for high spatial-temporal resolution imaging have been introduced1,2. DISCO (Differential Subsampling with Cartesian Ordering)3 is an under-sampled Cartesian sampling approach with pseudo-random k-space segmentation and a view-sharing reconstruction. It gives reliable fat suppression with 2-point-Dixon method. However, the dual echo acquisition prolongs echo spacing of TE/TR, resulting in extended scan time when high spatial-temporal resolution scanning is set up. In addition, fat-water separation reconstruction based on region growing requires high computation and memory usage that hinder from applying intensive reconstruction such as compressed sensing (CS) for further scan time acceleration. In this work, we propose a compressed sensing enabled time resolved imaging with fat frequency selective presaturation pulse (FatSAT).Methods
Randomly undersampled K-space points in ky-kz space are prepared for combined compressed sensing and parallel imaging. The k-space is segmented into annular regions consisting of the central region called A being fully sampled and the multiple outer regions called Bi (i =1-N, N: number of B regions) being sub-sampled. The outer region has different subsampling patterns giving pseudo-random distribution of k-space on Cartesian grid. Further segmentation of the k-space is performed such that each chemical fat suppression pulse is played out and followed by the acquisition of a segment of k-space views, which contains points from both central A region and outer Bi regions. After acquisition, each temporal phase dataset is composed of A region and multiple Bi regions from neighbor view sharing. In the reconstruction, randomly undersampled data on ky-kz space with Gaussian pattern for CS4 is reconstructed using L1-norm minimization of total variation forcing sparsity in an iterative manner to recover uniform k-space in parallel imaging domain. Finally data driven parallel imaging5 with auto-calibration signal (ACS) points produces complete k-space. A volunteer was scanned on a 3.0T Scanner (Discovery MR 750, GE Healthcare, Waukesha, WI, U.S.A.) with 32 channel body receiver array coil under IRB approval. The following scan parameters were used: Axial scan plane, 3D SPGR sequence, TR/TE = 3.5/1.2 msec, rBW = 90.9 kHz, Matrix = 320x320x52, FOV = 40x32 cm, slice thickness = 4.0mm, flip angle = 12 deg, number of temporal phase 3. Hyperbolic secant adiabatic pulse with 20 msec pulse width was used for chemical fat saturation pulse. Scan parameters were adjusted for the lowest net acceleration to scan within one breath hold. Image quality was evaluated with structural similarity index (SSIM)6 by selecting 10 slices for liver. The lowest accelerated image of PI alone was used as reference. Net acceleration is to be defined as #full sampling data / #undersampling data. Reconstruction time and maximum memory usage were measured for reconstruction performance to compare CS FatSAT DISCO to Dixon DISCO with the same scan parameter above on Intel@Xeon 2.5GHz, 20cores parallel computing, 99 GB memory and 64 bit linux.Results
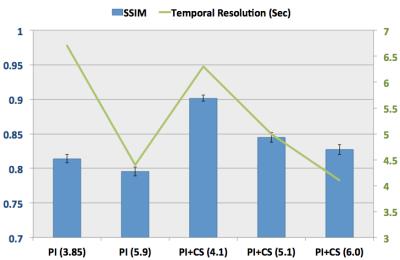
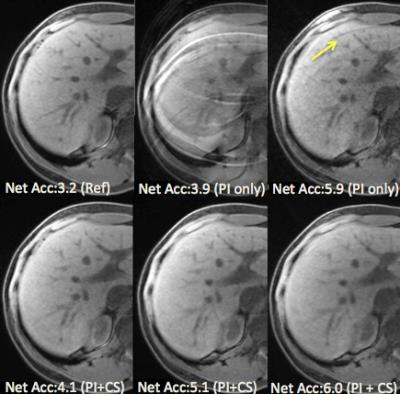
All the FatSat DISCO images were successfully acquired. Fig.1 shows the temporal resolution that is 6.7 sec, 4.4 sec, 6.3 sec, 5 sec and 4.1 sec and the SSIM value that is 0.82±0.006, 0.796±0.007, 0.91±0.004, 0.84±0.007 and 0.83±0.007 for PI (3.85), PI (5.9), PI+CS (4.1), PI+CS (5.1), and PI+CS (6.0), respectively. The value inside () shows net acceleration. Combined PI and CS outperformed PI alone for the similar acceleration comparison. Fig.2 shows representative images at PI alone and PI+CS. With higher acceleration, only PI image amplified noise and appeared unfolded artifact. On the other hand, combined PI+CS visualized slight image blurring. Total reconstruction computation time was 173.0 sec and 361.5 sec, maximum memory usage was 2.3GB and 9.1GB for PI+CS FatSat DISCO and PI Dixon DISCO, respectively.Discussions
This feasibility has been demonstrated that FatSAT DISCO with combined PI and CS can be clinically advantageous in terms of faster reconstruction time and lower memory usage than Dixon based DISCO that is already commercially available. In addition, combined PI and CS provides robustness to image artifact in large undersampled scanning with use of small parallel imaging reduction factor that may help in case of large coil geometry factor.Conclusion
We have developed FatSAT DISCO with reduced memory footprint, lighter compute demand and compatibility to CS technique to achieve highly accelerated scan time preserving image quality. Further clinical evaluation with dynamic scanning would validate the technique.Acknowledgements
No acknowledgement found.References
1. Salmani Rahimi M, Korosec FR, Wang K, Holmes JH, Motosugi U, Bannas P, Reeder SB. Combined dynamic contrast-enhanced liver MRI and MRA using interleaved variable density sampling. Magn Reson Med 2015;73:973–983.
2. Xu B, Spincemaille P, Chen G, Agrawal M, Nguyen TD, Prince MR, Wang Y. Fast 3D contrast enhanced MRI of the liver using temporal resolution acceleration with constrained evolution reconstruction. Magn Reson Med 2013;69:370–381.
3. Saranathan M, Rettmann DW, Hargreaves BA, Clarke SE, Vasanawala SS. Differential subsampling with cartesian ordering (DISCO): a high spatio-temporal resolution dixon imaging sequence for multiphasic contrast enhanced abdominal imaging. J Magn Reson Imaging 2012;35:1484–1492.
4. King K, Xu D, Brau AC, Lai P, Beatty PJ, Marinelli L. A New Combination of Compressed Sensing and Data Driven Parallel Imaging. In Proceedings of 18th Annual Meeting of ISMRM, Stockholm, Sweden, 2010. p. 4881.
5. Brau ACS, Beatty PJ, Skare S, Bammer R. Comparison of reconstruction accuracy and efficiency among autocalibrating data-driven parallel imaging methods. Mag Reson Med 2008;59:382–395.
6. Wang Zhou, Bovik, Alan C., Sheikh, Hamid R., and Simoncelli, Eero P.Image Qualifty Assessment: From Error Visibility to Structural Similarity. IEEE Transactions on Image Processing, Volume 13, Issue 4, pp. 600–612, April 2004.
Figures