5183
Spectrally selective spin-lock for fat-water imaging and simultaneous T1rho quantificationWeitian Chen1
1Imaging and Interventional Radiology, The Chinese University of Hong Kong, New Territory, Hong Kong
Synopsis
In conventional T1rho imaging, the fat signal is usually suppressed to avoid image artifacts and quantification errors. It is desirable to acquire both water and fat images for certain diseases. In this work, we present an approach to use spectrally selective spin-lock pulses to achieve T1rho quantification with simultaneous fat-water imaging. The theoretical analysis is provided with proof on numerical phantoms.
Purpose
Magnetic resonance T1rho relaxometry is a promising non-invasive imaging technology. In conventional T1rho imaging, the fat signal manifest as image artifacts and can induce quantification errors. Therefore, the fat signal is usually suppressed in T1rho imaging. However, there are several pathologies where direct visualization of fat is desirable, such as hepatic steatosis and other fatty infiltrative diseases. For these applications, separation of water and fat signals is highly desirable with the acquisition of both water image and fat image. In this work, we introduce a spectrally selective spin-lock pulse. The numerical simulations show this spin-lock pulse can be used to achieve T1rho quantification with simultaneous fat-water imaging. The theoretical analysis is provided with proof on numerical phantoms.Method
We use the spin-lock RF pulse cluster described in reference1 for the proposed application. Figure 1 shows the spectral profile of these RF pulse clusters. Note the spectral selectivity of the T1rho-prep RF pulse clusters at various time-of-spinlock (TSL). In contrast, the conventional RF pulse cluster does not possess spectral selectivity. To achieve water-fat separation and simultaneous T1rho quantification, a dual acquisition approach with a narrow bandwidth of T1rho-prep RF pulse cluster is designed such that the water signal is flipped to +z and –z, respectively, from two acquisitions, whereas the fat signal stays on +z from two acquisition. Consequently, the subtraction and summation of complex images from dual acquisition results in water and fat images, respectively. The water images are then fitted to a mono-exponential model for T1rho quantification. The same bandwidth of T1rho-prep is used for the dual acquisition to avoid signal deviation from mono-exponential T1rho decay after subtraction from two acquisitions1.Note the subtraction and summation cannot completely remove the fat and water signal when relaxation effect during the adiabatic pulse is non-negligible1. To address this, a scaling factor derived from Bloch simulation for the given T1rho-prep RF pulse cluster may be used to improve water-fat separation. The effectiveness of the proposed approach is proved with numerical phantoms experiments. The numerical phantoms consist of tubes of tissue of heart, white matter, grey matter, muscle, cartilage, liver, and fat. The data sets were simulated with Bloch equation. The simulation parameters include TSL 0, 10, 30, 60, 90ms, spin-lock frequency 500Hz, the adiabatic half passage (AHP) and reverse AHP used are hyperbolic secant pulse with duration 25ms. We conducted two numerical experiments. For the first experiment, the non-fat tissue and fat tissue are put in separate tubes. For the second experiment, the non-fat tissue and fat tissue are mixed in one tube. A variety of fat-to-water ratio is created in the second experiment. Conventional T1rho-prep and the proposed approach is conducted to compare the quantification accuracy at the presence of the various level of fat content.Results
Figure 2 and 3 show the results of the first numerical experiment. Note T1rho value can be accurately measured based on the proposed approach. Water-only and fat-only images are formed after subtraction and summation process. However, residual fat and water signal can be observed. This is due to non-negligible relaxation effect during the long adiabatic pulse which results in incomplete cancellation of undesired signal. Figure 4 shows the results of the second numerical experiment. When water and fat are mixed in one tube, the proposed approach results in an error of T1rho quantification, which is within 1% of the actual T1rho value. In contrast, the conventional T1rho-prep approach only works well when fat is completely suppressed. Significant quantification errors arise when the fat signal is not well suppressed.Discussion
We used numerical experiments as a proof of the effectiveness of the proposed method. Even though water/fat are not completely suppressed after sum/subtraction of two images, the residual error appears small. More importantly, the quantification of T1rho is robust using the proposed method. To obtain complete suppression of fat and water in water-only and fat-only images, a scaling of images before sum/subtraction is necessary when relaxation effect during the adiabatic pulse is non-negligible. The scaling factor may be derived from Bloch simulation. However, the scaling operation may be undesirable for T1rho quantification as it may cause water signal to deviate from mono-exponential decay after subtraction. Further analysis is also needed to evaluate the sensitivity of the proposed method to system imperfections, particularly off-resonance effect.Conclusion
A spectrally-selective constant amplitude spin-lock approach is proposed for quantitative T1rho imaging with simultaneous fat-water imaging. The theory is proved by numerial phantom experiments.
Acknowledgements
This study was supported by grants from the Research Grants Council of the Hong Kong SAR (Project No. SEG CUHK02).References
1. Chen W, Artifacts correction for T1rho imaging with constant amplitude spin-lock. Journal of Magnetic Resonance. doi: 10.1016/j.jmr.2016.11.002Figures

Figure 1. The spectral profile of the RF pulse clusters used for the proposed application. The RF pulse clusters used for the proposed dual-acquisition are the same as that used in reference (1). The left and right plot correspond to the spectral profile of these two RF pulse clusters. The right plot is the spectral profile of the conventional T1rho-prep with constant amplitude spin-lock.
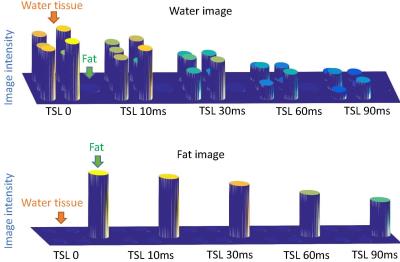
Figure 2. The water-only (top plot) and fat-only (bottom plot) images after water-fat separation process. All data sets are created with Bloch simulation. The images are rendered in 3D for better illustration of residual fat/water signal in water/fat images. From left to right are images acquired at five TSL 0, 10, 30, 60, 90ms. The water tubes include tissue of heart, grey matter, white matter, cartilage, muscle, and liver. The fat tube is separated from water tubes.
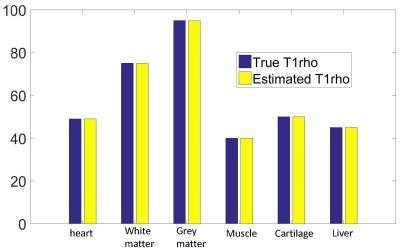
Figure 3. The actual T1rho value and the estimated T1rho value from the various tissue in the first numerical experiment. Note the proposed method can obtain accurate T1rho value when water tissue and fat tissue are in separate tubes.
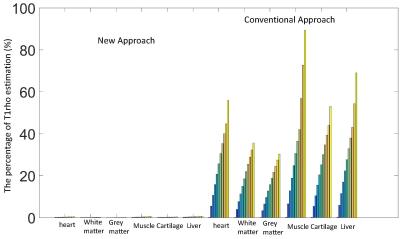
Figure 4. Percentage error of T1rho quantification of water tissue (defined as the T1rho error divided by the actual T1rho) when water and fat are mixed in one tube in the second experiment. The left and right six groups correspond to the measurement from the proposed and the conventional method, respectively. Within each group, there are 11 bars which correspond to the ratio of fat to water signal from 0 to 1 with 0.1 as increment. Note the conventional T1rho-prep only achieves accurate quantification when there is no fat signal. The proposed method can achieve reliable quantification without fat suppression.