5165
Improved SPIRiT Operator for Joint Reconstruction of Multiple T2-weighted Images1Electrical and Electronics Engineering, Bilkent University, Ankara, Turkey, 2National Magnetic Resonance Research Center, Bilkent University, Ankara, Turkey
Synopsis
Recently, compressed-sensing (CS) was proposed to jointly reconstruct undersampled multi-contrast datasets to exploit the common structural features therein. Here, we propose a method to improve joint reconstruction of multi-contrast acquisitions. Inspired by the SPIRiT framework for parallel imaging, our method linearly synthesizes missing data for each contrast from neighboring k-space data for all contrasts. To improve reconstruction quality, the proposed method high-pass filters calibration data to emphasize the weight of intermediate spatial frequencies in the interpolation operator. Phantom and in vivo results at 3T indicate that the proposed method outperforms reconstructions with conventionally estimated interpolators.
Introduction
Acquiring images with different tissue contrast often enhances the diagnostic information conveyed in an MRI exam. Yet, the increased scan time of multiple acquisitions prohibits common use of the multi-contrast approach. Recent reports proposed to overcome this limitation through compressed sensing (CS) 1-4, where undersampled multi-contrast datasets were jointly reconstructed to exploit the common structural features. However, intrinsic properties of k-space data (thus the validity of this assumption) may vary significantly with spatial frequency, leading to suboptimal reconstructions 5.
Here, we propose a method to improve joint reconstruction of multi-contrast acquisitions. Inspired by the SPIRiT framework for parallel imaging1, our method linearly synthesizes missing data for each contrast from neighboring k-space data for all contrasts. The interpolation operator that enables this synthesis is typically trained on fully sampled calibration data in central k-space. Yet shared information across contrasts predominantly reflect tissue boundaries, so such interpolators yield suboptimal data recovery towards the peripheries of k-space. To improve reconstruction quality, the proposed method high-pass filters calibration data to emphasize the weight of intermediate spatial frequencies in the interpolation operator. Phantom and in vivo results at 3T indicate that the proposed method outperforms reconstructions with conventionally estimated interpolators.
Methods
We adopted the SPIRiT framework (originally proposed for multi-coil data) to reconstruct multi-contrast acquisitions, to investigate the feasibility of improved reconstruction of images with different T2 weightings. To retain scan efficiency, the acceleration factor was taken to be equivalent to the total number of acquisitions. Acceleration was achieved through variable-density random sampling. The joint reconstruction of undersampled data was performed via the following optimization:
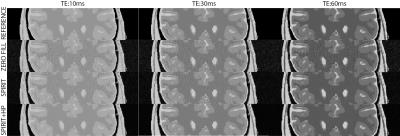
$$min\parallel DF\left\{m_{n}\right\}-y_{n}\parallel^{2}_{2}-λ\parallel (G-I){m_{n}}\parallel^{2}_{2}$$
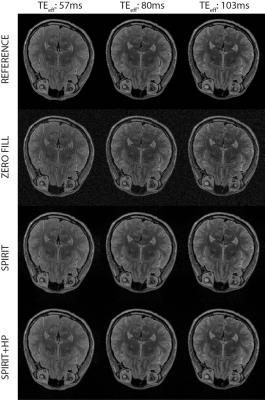
where mn is the reconstructed image for the nth contrast, is the acquired data, yn is the Fourier transform, D is the sampling pattern, G is the interpolation operator, and $$$λ$$$ is a penalty weight enforcing consistency with the calibration data. A conjugate gradient algorithm was used to solve the above problem. The interpolation kernel was 5x5, the calibration region was 58x58, and was 10-5, and 30 iterations were used. Conventional SPIRiT reconstructions were implemented with a regular interpolation operator. The operator was estimated by minimizing the calibration-consistency term based on the fully sampled central k-space data. The proposed reconstructions were then implemented with a modified operator, which was estimated by minimizing the calibration-consistency term after high-pass filtering the calibration data. Zero-filled Fourier (ZF), SPIRiT, and the proposed recontructions were compared on a numerical phantom and an in vivo dataset. The numerical phantom comprised three T2-weighted spin-echo acquisitions with TE = 10, 30, 60 ms; matrix size 424x362x88, 0.5 mm isotropic resolution. In vivo brain data were acquired on a healthy subject with the 3D SPACE sequences on a 3 T Siemens scanner. The following parameters were prescribed: 1 mm isotropic resolution, effective echo times of TEeff = 57, 80, 103 ms (actual echo times of 145, 221, 320 ms 7), 256x193x224 matrix size. Reconstruction quality was assessed by the peak signal-to-noise ratio (PSNR) metric, measured in reference to Fourier reconstructions of fully sampled data.
Results and Discussion
Visual inspection of numerical phantom results (Fig. 1) reveals that the proposed method (labeled as “SPIRIT+HP”) provides a close match to the reference images at all three TE values, while the remaining reconstructions show artifacts. Comparing PSNR values of the proposed reconstruction with the conventional SPIRiT reconstruction gives the following result: 30.60 dB vs. 24.19 dB at TE = 10 ms, 29.64 dB vs. 25.32 dB at TE = 30 ms, 28.03 vs. 25.84 dB at TE = 60 ms. Accordingly, the proposed method yields on average 4.3 dB better performance than conventional SPIRiT. Interestingly, the image with the shortest TE benefitted the most from the proposed reconstruction. Further simulations with different echo times (results not shown) also indicate this to be the case.
Figure 2 displays the in vivo T2-weighted acquisitions with three different echo times. While the differences are smaller than in the simulated brain phantom case, the proposed method performs better than the conventional SPIRiT reconstruction. The PSNR values were 21.77 dB vs. 20.21 dB at TEeff = 57 ms, 23.30 dB vs. 22.63 dB at TEeff = 80 ms, and 23.59 dB vs. 22.81 dB at TEeff = 103 ms.
Conclusion
Here, we propose a method to improve the performance of the SPIRiT operator for joint reconstruction of multiple T2-weighted images, via weighing the intermediate spatial frequencies in the interpolation operator. Our in vivo and simulation results demonstrate better data recovery and increased PSNR values for images with different contrast levels.Acknowledgements
This work was supported by the European Commission through FP7 Marie Curie Career Integration Grants (PCIG13-GA-2013-618834, PCIG13- GA-2013-618101), by a European Molecular Biology Organization Installation Grant (IG3028), by the Turkish Academy of Sciences through TUBA-GEBIP 2015 program, and by the BAGEP Award of the Science Academy.References
1. Bilgic B, Goyal VK, Adalsteinsson E. Multi-contrast reconstruction with Bayesian compressed sensing. Magn Reson Med 2011; 66:1601–1615.
2. Majumdar A, Ward RK. Accelerating multi-echo T2 weighted MR imaging: analysis prior group-sparse optimization. J Magn Reson 2011; 210:90–97.
3. Huang J, Chen C, Axel L. Fast multi-contrast MRI reconstruction. Magn Reson Imaging 2014; 32:1344–1352.
4. Gong E, Huang F, Ying K, Wu W, Wang S, Yuan C. PROMISE: parallel-imaging and compressed-sensing reconstruction of multicontrast imaging using SharablE information. Magn Reson Med 2015; 73:523–535.
5. Sung K, Hargreaves BA. High-frequency subband compressed sensing MRI using quadruplet sampling. Magn Reson Med 2013; 70:1306–1318.
6. Lustig, M. and Pauly, J. M. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn. Reson. Med. 2010;64(2):457–471.
7. Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn. Reson. Med. 2006;55(5):1030-7.
Figures