5164
Accelerated MR cardiac cine using TSPIRiT with generalized data fidelity1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, People's Republic of China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, People's Republic of China
Synopsis
MR cardiac cine plays a key role in quantifying the cardiac function. The measurement accuracy is highly dependent of both spatial and temporal resolution, which can be improved substantially by acceleration with parallel imaging. SPIRiT, a GRAPPA-like parallel imaging reconstruction, can be applied to cardiac cine by incorporating temporal sensitivity estimation (TSPIRiT). In this study, we propose to enhance MR cardiac cine using TSPIRiT with generalized data fidelity (GDF) based on the assumption that k-space signal in cardiac cine changes smoothly. Results show that the proposed method can provide better tradeoff between SNR and temporal resolution when compared with TSPIRiT and k-t SPIRiT.
Introduction
MR cardiac cine plays a key role in measuring the cardiac function. The measurement accuracy is highly dependent of both spatial and temporal resolution [1, 2], which can be increased by acceleration with parallel imaging. SPIRiT is a GRAPPA-like parallel imaging reconstruction, which uses all the neighborhoods for fitting and update k-space iteratively, and offers better noise performance over conventional GRAPPA [3]. SPIRiT can be applicable to cardiac cine in a frame-by-frame manner by incorporating temporal sensitivity estimation (TSPIRiT) [4]. Similar to k-t GRAPPA [5], SPIRiT can also be extended to k-t space, termed k-t SPIRiT. It has been shown to give higher accuracy than the frame-by-frame SPIRiT and k-t GRAPPA [6]. K-t SPIRiT utilizes a 3D interpolation kernel in k-t space and is very time consuming. Santelli, et al. proposed k-t ESPIRiT instead, which is more efficient in terms of reconstruction time. However, increased temporal blurring due to x-f support mask can be observed [7]. In this study, we propose to enhance TSPIRiT with generalized data fidelity (GDF) (TSPIRiT+GDF) based on the assumption that signal changes smoothly, which offers a better tradeoff between SNR and temporal resolution.Method
Theory
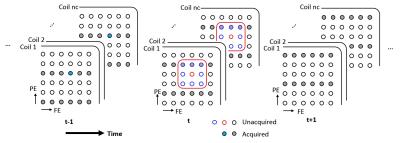
The proposed method (TSPIRiT+GDF) promotes consistency with calibration within current frame only, and it is more computationally efficient. Data fidelity enforces the reconstructed k-t space data to be consistent with acquisition. To reduce the noise associated with high undersampling factors, the concept of data fidelity is generalized based on the assumption that signal changes smoothly, i.e., an unacquired sample should be close to its temporally nearest samples (Figure 1). It can be expressed as an optimization problem.
$$\arg \min \limits_{x}||(G-I)x||_2^2+||λ(x-m)||_2^2$$
where G is a shift-invariant interpolation operator which is determined with calibration data. I is an identity matrix, x the vectorized k-t space data, m the nearest sampled points in temporal direction, λ the weighting vector that controls data fidelity. The weighting vector is adapted to the acquired data such that, when signal is changing very slowly, a wider window is used to further improve SNR. The weights can be determined with the cross-correlation between two frames.
Data Description and Retrospective Undersampling
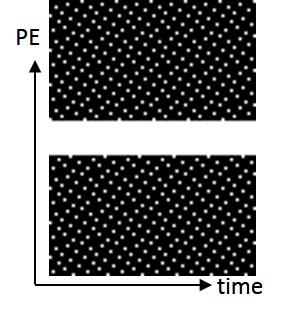
Data were obtained from online resources [8]. The retrospectively gated bSSFP short-axis view cardiac cine of a healthy volunteer was acquired using a 3T Siemens scanner with 30 channels, FOV=340×550 mm2, matrix size=168×416, slice thickness=6mm, resolution=2.02×1.32, 25 cardiac phases with a temporal resolution of 42.72 ms, TE/TR=1.78/3.56ms, and flip angle=40° [8]. Retrospective undersampling at R=6,8,10, and 12, with 8 central phase-encoding lines fully sampled were performed (Figure 2).
Image Reconstruction
TSPIRiT+GDF was incorporated with k-t space subtraction strategy, i.e., the temporal average was subtracted from each frame and reconstruction was applied to the residual k-t space. This has been adopted in [5, 9, 10], aiming to increase SNR of final images. Two methods were also implemented for comparison: (a) frame-by-frame SPIRiT (TSPIRiT) with low rank constraint (TSPIRiT+LR) with 3×3 kernel; and (b) k-t SPIRiT with 3×3×3 kernel [6]. For fair comparison, TSPIRiT+LR and k-t SPIRiT were also incorporated with k-t space subtraction, and 12 iterations were performed for all methods above (TSPIRiT+GDF, TSPIRiT+LR, and k-t SPIRiT).
Results
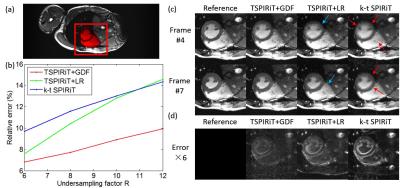
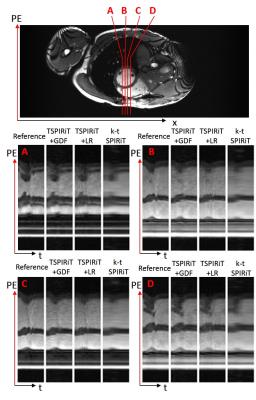
As shown in Figure 3(b), with increasing R, the difference in relative errors among different methods increased, indicating that the proposed TSPIRiT+GDF method could provide a more accurate reconstruction, especially at high undersampling factors. With k-t SPIRiT, certain structures were blurred due to cardiac motion (indicated by red arrows), while with TSPIRiT+LR, although we applied low rank constraint to improve SNR. The results suffered from SNR loss, and some image details (indicated by blue arrows) were buried under severe noise (Figure 3(c)). From temporal profiles (Figure 4), we found that k-t SPIRiT results were temporally more smoothed in comparison with other results, while TSPIRiT+LR results still sufferred from the noise enhancement.Discussion and Conclusions
With the proposed TSPIRiT+GDF method, SNR can be significantly improved without sacrificing temporal resolution, implying that it can achieve better tradeoff between SNR and temporal resolution than TSPIRiT+LR and k-t SPIRiT methods. Moreover, the weighting for generalized data fidelity is adaptively determined. During ejection and rapid inflow period when the ventricular volume changes drastically, and narrow window is used to reserve high temporal resolution. At end diastole when the signal changes very smoothly, a wide window is used aiming to achieve high SNR. This new method allows optimal tradeoff between SNR and temporal resolution for cardiac cine imaging throughout different cardiac phases.Acknowledgements
No acknowledgement found.References
[1] Y. L. Lu, K. A. Connelly, A. J. Dick, G. A. Wright, and P. E. Radau, "Automatic functional analysis of left ventricle in cardiac cine MRI," Quant Imaging Med Surg, vol. 3, pp. 200-9, Aug 2013.
[2] L. Feng, M. B. Srichai, R. P. Lim, A. Harrison, W. King, G. Adluru, et al., "Highly accelerated real-time cardiac cine MRI using k-t SPARSE-SENSE," Magn Reson Med, vol. 70, pp. 64-74, Jul 2013. [3] M. Lustig and J. M. Pauly, "SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space," Magn Reson Med, vol. 64, pp. 457-71, Aug 2010.
[4] H. Xue, Y. Ding, T.-C. Chang, C. Guetter, S. V. Raman, M.-P. Jolly, et al., "Improved real-time exercise stress cardiac cine imaging using self consistent parallel imaging with temporal sensitivity estimation (TSPIRIT)," Journal of Cardiovascular Magnetic Resonance, vol. 14, p. 1, 2012.
[5] F. Huang, J. Akao, S. Vijayakumar, G. R. Duensing, and M. Limkeman, "k-t GRAPPA: a k-space implementation for dynamic MRI with high reduction factor," Magn Reson Med, vol. 54, pp. 1172-84, Nov 2005.
[6] C. Santelli, T. Schaeffter, and S. Kozerke, "Radial k-t SPIRiT: autocalibrated parallel imaging for generalized phase-contrast MRI," Magn Reson Med, vol. 72, pp. 1233-45, Nov 2014.
[7] C. Santelli and S. Kozerke, "L1 k-t ESPIRiT: Accelerating Dynamic MRI Using Efficient Auto-Calibrated Parallel Imaging and Compressed Sensing Reconstruction," Journal of Cardiovascular Magnetic, 2016.
[8] M. Schloegl, M. Holler, A. Schwarzl, K. Bredies, and R. Stollberger, "Infimal convolution of total generalized variation functionals for dynamic MRI," Magn Reson Med, Aug 1 2016.
[9] J. Tsao, P. Boesiger, and K. P. Pruessmann, "k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations," Magn Reson Med, vol. 50, pp. 1031-42, Nov 2003.
[10] M. Blaimer, I. P. Ponce, F. A. Breuer, P. M. Jakob, M. A. Griswold, and P. Kellman, "Temporal filtering effects in dynamic parallel MRI," Magn Reson Med, vol. 66, pp. 192-8, Jul 2011.
Figures