5123
Effects of Load Induced Metabolic Changes of a Single Muscle on Whole Body Physiology1Medical Physics Group, Institute of Diagnostic and Interventional Radiology, Jena University Hospital - Friedrich Schiller University Jena, Jena, Germany, 2Institute of Physiotherapy, Jena University Hospital - Friedrich Schiller University Jena, Jena, Germany
Synopsis
31P MR spectroscopy enables a non-invasive evaluation of a metabolic response to a given exercise. A combination of this technique with other methods like 1H MRS, spirometry and blood lactate diagnostics improves the application field. Therefore, a broad range of metabolic parameters were acquired during an exercise of a single calf muscle to evaluate the effects of local energy demands on global parameters. We observed high adaptations resulting in good correlations between peripheral and locally measured values.
Purpose
Phosphorous and proton magnetic resonance spectroscopy (31P/1H-MRS) in loaded skeletal muscles enables non-invasive quantitation of exercise induced adjustments of energy metabolites, such as phosphocreatine (PCr), inorganic phosphate (Pi), as well as acidification, cytosolic buffering capacity, proton efflux or intermuscular lactate1. Spirometry and blood lactate diagnostics also provide insight into whole body metabolic adaptations but are only indirectly related to local energy demands in muscles during exercise. Therefore, in this study we applied a combination of MRS, spirometry and blood lactate diagnostics to evaluate the effects of local metabolic demands on global parameters.Methods
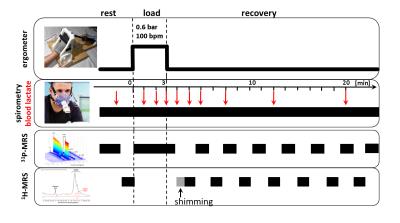
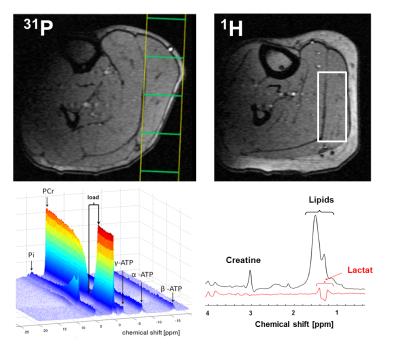
14 healthy male subjects (age: 27 ± 4 years) performed a 3 min plantar flexion with the right foot (0.6 bar pedal resistance, pedal frequency 100 bpm)2 within an 3 T MR scanner (Magnetom, PRISMA fit, Siemens, Erlangen, Germany). Spirometric data (VO2 and VCO2) were collected by using a commercial available spirometric system (PowerCube, Ganshorn, Medizin Electronic GmbH, Niederlauer, Germany). 31P- and 1H-MR spectra were acquired prior to, during and after the load (Fig. 1). In addition, blood lactate concentrations were quantified from blood samples, which were drawn from the right earlobe at defined time points in rest, during the load as well as during the recovery (Fig. 1). Interleaved series of 1H- and 31P-MR spectra were sampled with a double-tuned (1H/31P) flexible surface coil (RAPID Biomedical, Germany) in the m. gastrocnemius medialis by using a 2D FID-CSI sequence (TR/TE = 290/2.3 ms, 90° sinc rf excitation pulses, matrix: 8×8 voxels, slice thickness: 25 mm, voxel size: 25×25×25 mm³, temporal resolution: 8.4 s) and the 1H-MEGA-PRESS technique (TR/TE: 2000/140 ms, NEX: 16, editing of lactate CH2 spins at 4.1 ppm3). Locations of the selected CSI slice and MEGA-PRESS voxel are illustrated in Fig. 2. The MEGA-PRESS sequence was used to reduce contamination of the lactate doublet at 1.3 ppm by lipid resonances. The doublet was quantified by assuming a total creatine concentration of 44 mM1. All MR spectra were analyzed with the jMRUI 4.0 software (www.jmrui.eu). Spirometric data of VO2 recovery were fitted by using a bi-exponential fit. Overshoot of CO2 was determined by integrating the differences between the VO2 and VCO2 curves.Results
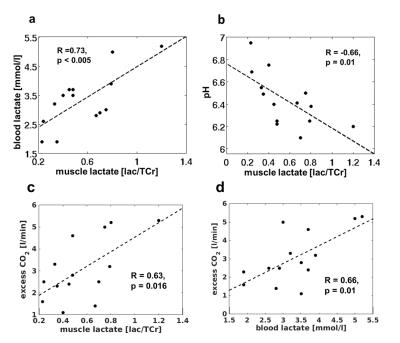
In all subjects, the load was associated with strong tissue acidification (end-exercise pH of 6.45 ± 0.19) as well as a PCr depletion below 20% followed by a noticeable slow PCr recovery (τPCr = 303 ± 141 s). Two minutes after the end of exercise, lactate concentrations revealed high inter-individual variation (59 ± 23 mM) and were negatively correlated with post-load pH values (R = -0.66, p = 0.01). The increase of inter-muscular lactate concentrations was also reflected in elevated blood lactate concentrations (pre-load: 1.4 ± 0.3 mmol/l; post-load: 3.1 ± 1,5 mmol/l). A significant positive correlation was determined between the post-load muscle and blood lactate concentrations (R = 0.73, p < 0.005). The O2 consumption and CO2 exhalation during the load phase attained 0.95 ± 0.31 l/min and 1.0 ± 0.37 l/min, respectively. After the load, a weak positive correlation was observed between the time rates of the PCr and VO2 recoveries (R = 0.44, p = 0.01). In all subjects, distinct CO2 overshoots were estimated (3.4 ± 1.3 l/min), which were positively correlated with post-load lactate concentrations in the muscle (R = 0.63, p = 0.016) as well as in the blood (R = 0.66, p = 0.01).Discussion and Conclusion
In this study, combined spectroscopic, spirometric and blood lactate measurements were applied to describe the local and global physiological adaptations to high intense muscular loads. Despite stressing only a small specific muscle group we were able to measure high respiration and blood lactate changes. Strong correlations between measured lactate concentrations in the periphery and in the muscle demonstrates the feasibility of 1H-MRS to quantify the load induced accumulation of lactate, which is a crucial exercise limiting parameter. As previously described, we also found a link between the recovery rates of aerobic PCr store refilling and global oxygen demand after the load4. Based on the demonstrated advantages of such combined measurements, our future studies will focus on training specific physiological adaptations in order to provide a more differentiated view on training efficiency aspects.Acknowledgements
This work was supported by the Competence Centre for Interdisciplinary Prevention (KIP) at the Friedrich Schiller University Jena and the German Professional Association for Statutory Accident Insurance and Prevention in the Foodstuffs Industry and the Catering Trade (BGN). K.M. is supported by a graduate scholarship of the Friedrich-Schiller-University Jena (Landesgraduiertenstipendium). K.M. also acknowledges support by the German Academic Exchange Service (DAAD) for a short-term international scholarship at the University of Liverpool (57044996). The authors declare to have no relevant financial interests to disclose with regard to this study.References
1 Ren J et al. Noninvasive monitoring of lactate dynamics in human forearm muscle after exhaustive exercise by 1H-magnetic resonance spectroscopy at 7 tesla. Magn Reson Med. 2013; 70(3): 610-9.
2. Tschiesche K et al. MR-compatible pedal ergometer for reproducible exercising of the human calf muscle Med Eng Phys. 2014; 36(7): 933-37.
3. Tschiesche K et al. Multimodal determination of load changes in the muscle - A combination of 1H-MEGA-PRESS and blood sampling.Proc ISMRM, vol. 23,2015
4. Rossiter HB et al. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans J Physiol. 1999; 518(3): 921-32.
Figures