5109
Improved delineation of air-bone interface in in-vivo high-resolution bright bone ZTE MRI at 3T1Mayo Clinic, Rochester, MN, United States, 2Mayo Clinical, Rochester, MN, United States, 3GE Healthcare
Synopsis
Using MRI for depicting solid cortical bone structures is of increasing clinical interest. Due to its low water content and short transverse relaxation time, cortical bone appears as signal void in conventional gradient echo or spin echo pulse sequences. This allows “black bone” techniques to be used when air does not confuse the visualization of cortical bone. In cases differentiation between bone tissues and ai are desired “bright bone” techniques utilizing Ultrashort echo time (UTE) or Zero TE (ZTE) MRI-have been proposed. Long T2-suppression methods (e.g., echo subtraction, long T2 saturation) are often applied to generate positive cortical bone contrast. However, clinical applications of these methods are still limited due to significant increase in acquisition time and reduced SNR efficiency. Recently a prototype proton density (PD)-weighted, zero TE (ZT) sequence has been demonstrated clinically. This work aims to improve the bright bone MRI using the ZTE sequence by optimizing the bone signal during data acquisition, minimizing partial volume effect with ultra high resolution data acquisition and optimizing the image processing for better bone/air differentiation.
Introduction
Using MRI for depicting solid cortical bone structures is of increasing clinical interest. Due to its low water content (~20%) and short transverse relaxation time (T2* ~ 0.4ms), cortical bone appears as signal void in conventional gradient echo or spin echo pulse sequences. This allows “black bone” techniques to be used when air does not confuse the visualization of cortical bone (1). In cases differentiation between bone tissues and ai are desired “bright bone” techniques utilizing Ultrashort echo time (UTE) or Zero TE (ZTE) MRI-have been proposed (2, 3). Long T2-suppression methods (e.g., echo subtraction, long T2 saturation) are often applied to generate positive cortical bone contrast. However, clinical applications of these methods are still limited due to significant increase in acquisition time and reduced SNR efficiency. Recently a prototype proton density (PD)-weighted, zero TE (ZT) sequence has been demonstrated clinically (4). This work aims to improve the bright bone MRI using the ZTE sequence by optimizing the bone signal during data acquisition, minimizing partial volume effect with ultra high resolution data acquisition and optimizing the image processing for better bone/air differentiation.Methods
Imaging experiments were performed using the ZTE sequence on a Discovery MR750w 3.0T scanner (GE, Waukesha, WI). IRB approval was obtained for all healthy human studies. Common acquisition parameters included FOV/slice thickness/BW= 24cm/1mm/83.3KHz. The highest flip angle of 2° was used (limited by the RF pulse duration required to achieve wide excitation bandwidth to minimize image blurring due to excitation profile). To minimize the partial volume effect from surrounding tissues, readout matrix sizes of 384×384×240 and 512×512×240 were used. The acquisition times were ~12 and 9 minutes for the two matrix sizes respectively. Image reconstruction was performed using the offline reconstruction using vendor-supplied software. Both the original magnitude images and the processed bone images were generated. The original magnitude images were subsequently processed offline using Matlab code to improve the bone/air differentiation. First, signal intensity correction was performed on a slice-by-slice basis by normalizing it to the mean soft tissue signal in that slice. Second, a multi-resolution ROI based intensity correction was applied to further correct for residual signal intensity variations in a way similar to (3). A mask was then generated based on the histogram and applied to the signal intensity inverted images to obtain the final images.Results and Discussion
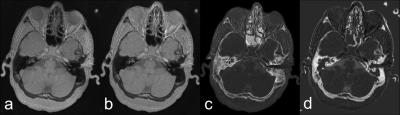
Representative signal intensity histograms with and without intensity correction are shown in Fig. 1. The highest peak corresponds to the noise while the broader peak with high signal intensity (higher bin index) represents the soft tissue. Between the two peaks is the cortical bone signal. The improvement in the signal distribution with the intensity correction is clearly demonstrated before (Fig1a) and after slice based intensity correction (Fig. 1b) and ROI based intensity correction (Fig. 1c). Fig. 2 shows a representative section of the head of a volunteer. The uncorrected magnitude images (Fig. 2a), primarily dominated by PD contrast, reveal a slight bottom-to-top gradient in signal intensity. The intensity corrected image (Fig. 2b) shows more homogeneous intensities across the FOV. The difference in signal intensity among air, tissue and cortical bone is readily visible. The bone image (Fig. 2C) generated automatically by the scanner depicted the bone nicely but it didn’t differentiate the cortical bone from the air very well, as can be seen in the air cavity in the nose. Air is suppressed much better in the image obtained with the method proposed in this work (Fig. 2d). Fig 2. Representative image from the same section. (a) Magnitude image. (b) Intensity corrected magnitude image (c) Bone image generated automatically using the program provided by the manufacturer. (d) Bone image created using the proposed method. Fig. 2 Representative magnitude (a), phase (b) and merged composite images. (c). Due to the relatively long acquisition time, a certain degree of motion artifact is present in some of the images despite the inherit resilience of ZTE sequence to motion. These artifacts could be potentially minimized by sampling the k-space in an interleaved fashion and then using low resolution images generated from each interleave for rigid body motion correction.Conclusion
With optimized high resolution ZTE MR imaging and improved image processing, high quality cortical bone imaging of the human head has been demonstrated on a clinical 3T scanner. The goal is to potentially use this method in applications such as attenuation correction PET/MR, one-stop neurosurgical planning and radiation dose reduction in pediatric patients.Acknowledgements
No acknowledgement found.References
1. Eley et al., Br J Radiol 2012; 84:272-278. 2. Du et al., NMR Biomed 2013; 26:489-506. 3. Wehrli F. J Magn Reson 2013; 229:35-48. 4. Wiesinger et al., Magn Reson Med 2016; 75:107–114.Figures