5103
Feasibility of Simultaneous Bilateral Knee Imaging with a Dual-Coil Setup1Radiology, Stanford University, Stanford, CA, United States
Synopsis
Osteoarthritis (OA) is commonly a bilateral disease. While long scan time and costs have precluded separate scanning of both knees in clinical MRI, there is evidence that bilateral examinations are beneficial for evaluation of OA changes, especially for longitudinal studies. In this study, we demonstrate the feasibility of simultaneously imaging both knees with similar scan time, SNR, and quantitative accuracy compared to single knee acquisitions.
INTRODUCTION
Osteoarthritis (OA) remains a tremendous burden to society, affecting the majority of the population by age 65 [1]. OA is commonly a bilateral disease. Research studies of OA, including the Osteoarthritis Initiative (OAI), often evaluate both knees. Unfortunately, despite the promise of MRI to detect early OA changes in cartilage, scan time restrictions often limit the MRI protocol. Extended MRI protocols result in long scan times, which increase costs and patient motion, and can affect patient retention in longitudinal studies. Therefore, simultaneous imaging of both knees without added scan time could drastically reduce scan costs, improve patient comfort and retention, and eliminate potential parameter/sequence differences between scans of each knee. In this work, we evaluate the feasibility of imaging both knees simultaneously with similar scan time, SNR, and quantitative accuracy compared to single knee acquisitions.METHODS
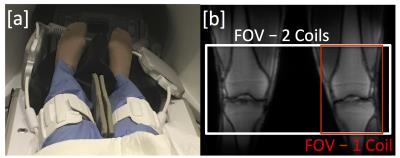
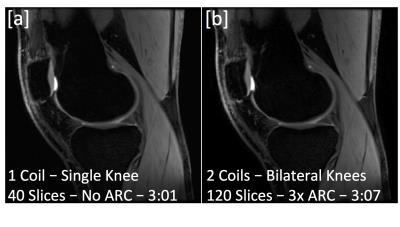
Hardware modifications and safety testing were performed to enable MR imaging with two 16-channel flexible phased-array receive only knee coils (NeoCoil, Pewaukee, WI). To demonstrate feasibility, both knees of a healthy volunteer were scanned with the dual-coil configuration (Fig 1a). Imaging was performed with a quantitative double-echo steady-state (qDESS) sequence [2,3] in the sagittal plane with parameters: FOV=16 cm, Matrix: 256x256, Slice Thickness: 3 mm, TR/TE1/TE2: 17.7/5.1/30.3 ms, Slices: 40 (Unilateral), 120 (Bilateral), Scan Time: 3:01 (Unilateral), 3:07 (Bilateral). For comparison, one coil was removed and the subject was rescanned in the conventional one-coil, one-knee configuration. In the bilateral configuration, the number of sagittal slices was increased 3-fold to include both knees in the field of view (Fig. 1b). To maintain scan time compared to a single knee acquisition, parallel imaging and reconstruction with ARC (Autocalibrating Reconstruction for Cartesian imaging) was used to accelerate by a factor of 3 in the slice direction. T2 mapping was performed by fitting qDESS images to complex signal models [2,3]. T2 maps were compared between bilateral and unilateral acquisitions. Additionally, parallel imaging noise amplification was characterized with coil (geometry) g-factor maps [4] with sensitivity maps computed using ESPIRiT [5].RESULTS
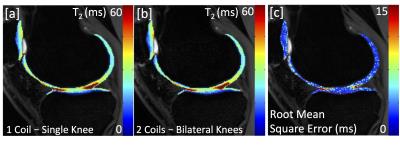
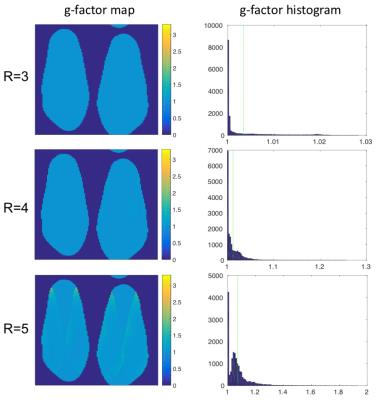
Unilateral and bilateral knee scans acquired in similar scan times showed similar image contrast and detail (Fig. 2). Additionally similar T2 relaxation times were observed (Fig. 3a,b). An average pixel-by-pixel root mean squared error (RMSE) of 3.1 ms was observed in cartilage T2 maps between unilateral and bilateral scans (Fig. 3c), which is comparable to scan-rescan reproducibility of unilateral scans (2.2 ms). g-factor maps with an acceleration factor of R= 3, 4, 5 are shown in Figure 4.DISCUSSION
This preliminary work demonstrates the potential for a bilateral coil setup with parallel imaging to scan both knees with similar scan times and SNR to a unilateral acquisition. The g-factor reflects a coil’s ability to perform parallel imaging acceleration. With R=3, the acquisition time between unilateral and bilateral acquisitions was roughly the same (bilateral scans: 3x more z phase encodes (slices-120:40), 3x accelerated, with extra time to acquire ARC calibration region). Thus, the g-factor of close to 1 means that SNR should be unaffected between acquisitions, as was evidenced. In general, the g-factors were low for all acceleration factors tested, suggesting that further acceleration could be used. Additionally, the equivalence in T2 maps between acquisitions suggests that bilateral acquisitions can be used for quantitative mapping as well. Further testing is needed to prove that bilateral protocols are reproducible and provide equivalent quantitative information to single knee acquisitions.CONCLUSION
This work shows the feasibility of simultaneous scanning both knees with a bilateral coil setup and parallel imaging in similar scan times and SNR to conventional unilateral knee scans. Simultaneous bilateral knee scanning has the potential for broad application, both for OA research and for routine clinical scanning.Acknowledgements
We would like to acknowledge our funding sources: GE Healthcare, NIH R01EB002524, NIH R01AR0063643 and NIH K24AR062068.References
1. Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Practice & Research Clinical Rheumatology 2006;20(1):3-25.
2. Jordan CD, Monu UD, McWalter EJ, Watkins RD, Chen W, Bangerter NK, Hargreaves BA, Gold GE. CubeQuant T1rho, qDESS T2, and cones sodium measurements are sufficiently reproducible for in vivo cartilage studies. 2013 2013; Salt Lake City. p 436.
3. Staroswiecki E, Granlund KL, Alley MT, Gold GE, Hargreaves BA. Simultaneous estimation of T(2) and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3 T. Magn Reson Med 2012;67(4):1086-1096.
4. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magnetic Resonance in Medicine 1999;42(5):952-962.
5. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med 2014;71(3):990-1001.
Figures