5090
GRAPPATINI put to use: How MSK applications benefit from highly undersampled T2 mapping and synthetic contrastsMarcus Raudner1, Tom Hilbert2,3,4, Tobias Kober2,3,4, Vladimir Juras1, Ewald Moser5, Claudia Kronnerwetter1, David Stelzeneder6, and Siegfried Trattnig1
1High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 3Department of Radiology, University Hospital (CHUV), Lausanne, Switzerland, 4LTS5, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 5Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 6Department of Orthopaedics, Medical University of Vienna, Vienna, Austria
Synopsis
The quantitative measurement of the T2 relaxation time has been shown to be a useful tool for radiological diagnosis. However, the use of quantitative MRI (qMRI) in clinical routine is often hindered due to long acquisition times. Here, we assess T2 parameters in the lumbar and cervical spine as well as the knee using GRAPPATINI, a model-based accelerated T2 mapping sequence. Additionally, synthetic T2-weighted (T2w) images are derived from the quantitative maps. The T2 maps and synthetic T2w images are compared to conventional T2w and T2 mapping sequences, yielding an overall 5.8-fold time-saving.
Purpose
T2 mapping offers great potential as non-invasive tool in musculoskeletal applications since T2 relaxation times differ between healthy and diseased cartilage. This has already been shown in studies assessing knee cartilage and intervertebral discs (IVDs)2–6. However, the long acquisition times of conventional Carr-Purcell-Meiboom-Gill (CPMG) sequences prohibits the broad clinical application of T2 mapping. Therefore, intermediate-weighted (IW), T2w and T1w TSE sequences are used for morphological evaluation of the knee7 and the IVD8 providing only qualitative images for diagnosis. GRAPPATINI1 is a model-based iterative algorithm to reconstruct highly-undersampled k-space data and offers a T2 map and multiple synthetic T2w images with arbitrary echo times directly reconstructed on the scanner. This enables fast T2 mapping acquisition embedded in a clinical workflow. Here we compare GRAPPATINI to a fully sampled CPMG sequence and TSE images acquired with a clinical protocol in a healthy volunteer through the assessment of knee cartilage as well as lumbar and cervical IVDs.Methods
After written and oral consent was obtained, one healthy volunteer (age 22 years, female) was scanned at 3T (Magnetom Prisma, Siemens Healthcare, Erlangen, Germany). GRAPPATINI was used to acquire 10-fold undersampled 2D k-space data. For comparison, standard fully-sampled CPMG T2 mapping and a clinical TSE protocol were acquired. A dedicated 15-channel knee coil, 64-channel head/neck coil and an 8-channel spine coil in conjunction with a 32-channel body coil were used as receivers. T2 maps were acquired from CPMG data using the MapIt product software installed on the scanner. T2 map and synthetic contrasts from GRAPPATINI were directly reconstructed on the scanner hardware. TSE and synthetic contrasts were compared at identical TE regarding their respective signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR). An overview of the employed sequence parameters is shown in figure 2. T2 values in regions-of-interest (ROI) were assessed using JiveX (Visus Technology Transfer GmbH, Germany) and analyzed via paired t-tests and calculation of Pearson correlation coefficients. Knee cartilage T2 values were aggregated from anterior, central and posterior femoral cartilage as well as patellar and tibial regions. IVDs were compared in the regions of the anterior and posterior annulus fibrosus (AF) as well as the nucleus pulposus (NP). ROIs were manually drawn and copy-pasted between maps.Results
Figure 1 shows a side-by-side comparison of T2w TSE and synthetic images with a color-coded overlay of the T2 maps derived via CPMG and GRAPPATINI. SNR was compared for bone, cartilage and muscle and CNR for cartilage-bone, cartilage-muscle and cartilage-fluid. Comparing these parameters for the synthetic conrasts and the T2w TSE images, SNR and CNR correlated significantly (r=.962,p<.001 and r=.995,p<.001) and were not significantly different (p=.221 and p=.329). The mean T2 values in CPMG and GRAPPATINI maps correlated significantly in the lumbar spine (r=.984,p<.001), cervical spine (r=.838,p<.001) and the knee (r=.928,p<.001). This shows strong coherence of both methods of relaxation time measurement. For an overview of the assessed mean values see Figure 3. Mean values of the respective areas differed significantly in the knee (p=.010) and the cervical (p<.001), but not the lumbar spine (p=.112).Discussion
The strong Pearson correlation coefficients of CPMG and GRAPPATINI suggest that this prototype sequence could potentially replace the fully sampled standard approach. High k-space undersampling for T2 mapping allows for a significant shortening in scan time required in the knee (2:14min vs. 12:54min) and the cervical and lumbar spine (2:32min vs. 13:38min) while preserving data quality and accuracy of generated T2 maps. GRAPPATINI tends to overestimate in areas with low T2 values because the algorithm requires at least a factor of three times ∆TE for an accurate reconstruction. However, CPMG and a typical mono-exponential model tend to overestimate T2 in areas with higher values, whereas GRAPPATINI has already been shown to be very close to a single-echo reference in these T2 value areas.1 For this preliminary assessment of the cervical spine, GRAPPATINI suffered from continuous overestimation due to image artifacts, but still provided a highly significant correlation to CPMG-based T2 values.Conclusion
GRAPPATINI makes way for accurate assessment of T2 relaxation times and additionally provides synthetic T2w TSE-like contrasts comparable to conventional morphological sequences. Using GRAPPATINI, quantitative (T2 mapping) and qualitative (synthetic T2w TSE) assessment of anatomical regions is possible in less than 3 minutes at identical spatial resolution. Acquiring quantitative high-resolution images within clinically feasible scan times might increase the value of T2 mapping and encourages early diagnosis of disease based on quantitative, automated analysis. This is a promising outlook since broad clinical application of qMRI may allow establishing a database of various normative ranges to detect changes in early stages of disease.Acknowledgements
This work was supported by the Austrian Science Fund (FWF) KLI541-B30References
1.Sumpf, T. J., Uecker, M., Boretius, S. & Frahm, J. Model-based nonlinear inverse reconstruction for T2 mapping using highly undersampled spin-echo MRI. J. Magn. Reson. Imaging 34, 420–428 (2011). 2.Kijowski, R., Blankenbaker, D. G., Munoz Del Rio, A., Baer, G. S. & Graf, B. K. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology 267, 503–13 (2013). 3.Dunn, T. C., Lu, Y., Jin, H., Ries, M. D. & Majumdar, S. T2 Relaxation Time of Cartilage at MR Imaging: Comparison with Severity of Knee Osteoarthritis. Radiology 232, 592–598 (2004). 4.Trattnig, S. et al. Lumbar intervertebral disc abnormalities: Comparison of quantitative T2 mapping with conventional MR at 3.0 T. Eur. Radiol. 20, 2715–2722 (2010). 5. Mayerhoefer, M. E. et al. Quantitative analysis of lumbar intervertebral disc abnormalities at 3.0 Tesla: value of T(2) texture features and geometric parameters. NMR Biomed 25, 866–872 (2012). 6. Welsch, G. H. et al. Parametric T2 and T2* mapping techniques to visualize intervertebral disc degeneration in patients with low back pain: Initial results on the clinical use of 3.0 Tesla MRI. Skeletal Radiol. 40, 543–551 (2011). 7. Peterfy, C. G., Schneider, E. & Nevitt, M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis and Cartilage 16, 1433–1441 (2008). 8. Majumdar, S. Magnetic resonance imaging and spectroscopy of the intervertebral disc. NMR Biomed. 19, 894–903 (2006).Figures
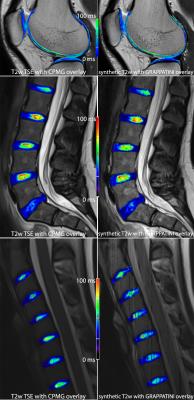
left:
T2w TSE contrasts with a color-coded T2 map overlay cumulatively sampled in
12:54 min (knee) and 14:38 (spine) in total
right: synthetic contrasts with color-coded T2 map overlay derived from GRAPPATINI
in a total scan time of 2:14 min (knee) and 2:32 (spine)
relevant
protocol parameters used in the three body regions to acquire T2 weighted
images and T2 maps with a fully sampled CPMG and a GRAPPATINI sequence
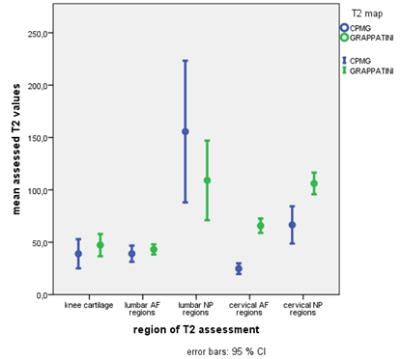
mean
T2 values with error bars sorted by region – GRAPPATINI tends to present
slightly higher values in lower (< 50 ms) regions, but shows solid in higher
value regions, wheres CPMG is overestimaging in regions like the lumbar NP. The
bias for the cervical assessment is clearly visible but seems to be an absolute
offset while showing significant correlation of T2 values
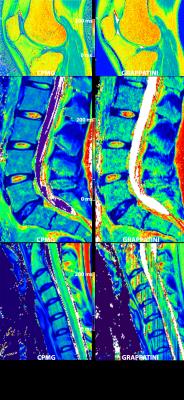
color-coded T2 maps from CPMG and GRAPPATINI (10-fold undersampled) in a side-by-side comparison