5080
Optimization of brain extraction increases global cortical thickness accuracy1Department of Computing and Mathematics, University of Sao Paulo, Ribeirao Preto, Brazil, 2Department of Neuroimaging, King's College London, London, United Kingdom, 3Department of Forensic & Neurodevelopmental Sciences, King's College London, London, United Kingdom
Synopsis
Reconstruction of the cortical surface is of great importance as a biomarker for many brain diseases, and in recent years a number of advances in image processing and analysis have been made in the cortical reconstruction process. However, despite the scientific community employing a range of advanced surface reconstruction algorithms in order to improve quantitative accuracy, cortical thickness measurement is still a challenge. Here, we address the question of whether a better brain extraction procedure can improve the cortical surface reconstruction. Our analyses suggest that a more accurate brain mask directly affects the global cortical thickness estimate, reducing its quantitative uncertainty.
Purpose
One of the most important biomarkers based on structural characteristics of the brain is cortical thickness, which plays an important role in several areas, including Alzheimer1-3, schizophrenia4, brain aging5-7 and others8-10. Recently, efforts have been made to provide accurate and reliable cortical reconstruction algorithms, with the Freesurfer11 package being probably the most well-known image analysis software widely used for this purpose in the scientific community.
However, cortical surface extraction still presents considerable problems, which can lead to over or
In the current work, we focus on the brain extraction procedure, in order to study whether a more precise brain mask can increase the accuracy of the cortical surface and thickness measurements.
Methods
A total of 8 healthy individuals (5 men, age min/max = 50/75 years) were scanned on 3 separate occasions (0, 1 and 4 weeks), resulting in a total of 24 3D T1-weighted (T1w) MRI images. Scanning was performed on a 3T scanner (General Electric, MR750, NIHR Wellcome King's Clinical Research Facility) with the following imaging parameters: inversion recovery prepared spoiled gradient echo (IR-SPGR), voxel size of 1.055x1.055x1.2 mm, FOV=270 mm, flip angle=11o, TE/TR/TI=3.0/7.3/400 ms, matrix size of 256x256.
The five most common brain extraction methods (3dSkullStrip (AFNI)14, BET (FSL)15, optiBET16, ROBEX17, and Freesurfer native method11) were investigated here, with no pre-processing being carried out on the T1w images in order to preserve the same initial conditions. Manual brain volume segmentations were obtained by careful editing of the boundary between the gray matter external surface and non-brain tissues; this manual delineation was adopted as the “ground truth” against which each automatic brain extraction method was compared. Each brain extraction algorithm was applied using its optimum parameters, as described in the literature8,9.
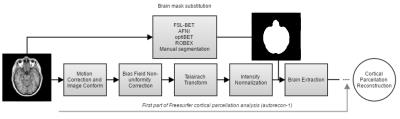
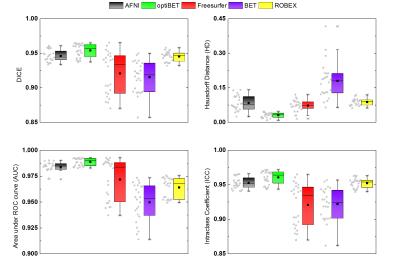
Each brain volume was evaluated based on the Dice similarity index (DICE10), Hausdorff Distance (HD11), Area Under ROC (receiver operating characteristic) curve (AUC12), and Interclass Correlation (ICC13) in order to define the most robust brain extraction method. Based on these results, the two best performing automatic brain extraction methods were selected and these were used to perform a global cortical reconstruction analysis for both brain hemispheres (Figure 1), which were then compared with the one from the Freesurfer standard procedure. The reconstructed surfaces were visually inspected for the obvious difference from the ground truth, and the absolute error (AE) between the surfaces was calculated at each vertex of the grouped normalized cortical thickness mesh from all the subjects.
Results
Figure 2 illustrates the robustness of each automatic brain extraction method. Each image similarity metric highlights a specific characteristic of each procedure, where, ideally, the DICE, ICC, and AUC metrics should be maximized and HD should be minimized. From this evaluation, the optiBET and AFNI methods were selected (along with Freesurfer) to perform the full cortical parcellation procedure.
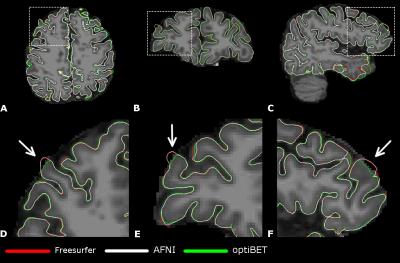
Figure 3 summarizes the AE values obtained in the global analyses and Figure 4 shows an example of cortical surface delineation errors. In general, the optiBET brain extraction method showed a considerable decrease in the global AE and better definition of the gray matter surface shape.
Discussion and Conclusion
As seen in Figure 3 and 4, the total number of large errors is reduced when a more robust brain extraction method is adopted. Simple adopting an alternative brain extraction method shows promise for more accurate cortical pial surface and thickness measurements. Our results indicate an impact over the parietal and temporal lobes, which could be valuable for brain analyses that depend on cortical thickness measures in those areas. Minimizing the chance of propagating voxels that do not belong to gray matter, by using a more realistic brain mask, can help to minimize error propagation to the final stage of cortical surface reconstruction.
In this study, the impact of the brain extraction procedure on the cortical surface reconstruction was demonstrated, which we hope will raise awareness in the scientific community about the direct influence of data pre-processing on the accuracy of cortical thickness evaluation. Further ongoing improvements in the data pre-processing steps in Freesurfer are likely in future software updates, but we recommend that, unless and until such updates address the brain extraction step, researchers should consider using alternative approaches such as those we have tested here.
Acknowledgements
The authors would like toReferences
1. Hartikainen P, Räsänen J, Julkunen V, Niskanen E, Hallikainen M, Kivipelto M, et al. Cortical thickness in frontotemporal dementia, mild cognitive impairment, and Alzheimer’s disease. J Alzheimers Dis. 2012;30(4):857–74.
2. Hampel H, Prvulovic D, Teipel S, Jessen F, Luckhaus C,
3. Querbes O, Aubry F, Pariente J, Lotterie J-A, Démonet J-F, Duret V, et al. Early diagnosis of Alzheimer’s disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132(Pt 8):2036–47.
4. Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–88.
5. Hanford LC, Nazarov A, Hall GB, Sassi RB. Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord. 2016;18(1):4–18.
6. Kini LG, Gee JC, Litt B. Computational analysis in epilepsy neuroimaging: A survey of features and methods. NeuroImage Clin. 2016;11:515–29.
7. Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, et al. Cerebral cortex and the clinical expression of Huntington’s disease: complexity and heterogeneity. Brain. 2008;131(Pt 4):1057–68.
8. Popescu V, Battaglini M, Hoogstrate WS, Verfaillie SCJ, Sluimer IC, van Schijndel RA, et al. Optimizing parameter choice for FSL-Brain Extraction Tool (BET) on 3D T1 images in multiple sclerosis. Neuroimage. 2012;61(4):1484–94.
9. Iglesias JE, Cheng-Yi Liu, Thompson PM, Zhuowen Tu. Robust Brain Extraction Across Datasets and Comparison With Publicly Available Methods. IEEE Trans Med Imaging. 2011;30(9):1617–34.
10. Dice LR. Measures of the Amount of Ecologic Association Between Species. Ecology. 1945;26(3):297–302.
11. Taha AA, Hanbury A. An efficient algorithm for calculating the exact Hausdorff distance. IEEE Trans Pattern Anal Mach Intell. 2015;37(11):2153–63.
12. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36.
13. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8.
Figures