5079
Improved 23Na MRI Quantification of the Human Brain at 3T Using Partial Volume Correction Techniques1Department of Radiology, University of Melbourne, Melbourne, Australia, 2Department of Radiology, University of Melbourne
Synopsis
In this study we have focused on the development and application of PVC techniques for improving the quantification accuracy of STC with 23Na MRI at 3T. Although PVEs are known to induce errors in quantification, it has not been widely used in 23Na MRI. While different PVC algorithms have been proposed in the PET literature, each method has its limitations and relies on simplified assumptions. The proposed hybrid methods, such as GTM+RBV, aimed to overcome such limitations are most robust.
Purpose
Recent development of MRI data acquisition techniques has made it clinically feasible to conduct whole-brain 23Na MRI at 3T and assess sodium tissue concentration (STC). However, the accuracy of STC quantification by 23Na MRI at 3T is still hampered by the limited spatial resolution. The normal spatial resolution is typically in the range of 5-6 mm and the full width at half maximum (FWHM) of the actual point spread function (PSF) is even larger. The purpose of this study is to optimize partial volume correction (PVC) using anatomical information from high-resolution 1H MRI for post-reconstruction correction of 23Na MRI to improve the quantification accuracy.Materials and Methods
The MRI experiments were performed on a Siemens 3T trio system using a custom-built dual-tuned 1H/23Na birdcage coil tuned at 123.2 and 32.6 MHz, respectively. T1-MPRAGE 1H MRI was acquired using a standard clinical protocol. 23Na MRI was performed using the flexible twisted projection imaging (FlexTPI) method (1,2) with the following acquisition parameters: FOV=240mm, matrix size=44, NEX=2, total imaging time=8min, Readout duration Tadc=11ms, TE/TR=0.3/160ms, flip angle=90. The protocol included also routine B0 and B1 mappings. The 23Na MRI data were processed as described previously to compute B0 and B1 corrected TSC images (3). We studied 93 adult volunteers (25-91 years old, male/female= 32/61) in ~3 years. Three different PVC algorithms previously proposed for correction of PET data (4-6) were adopted to construct hybrid methods for PVC of 23Na MRI including the regional based geometric transfer matrix (GTM), voxel-based multi-target correction (MTC) and region-based voxel-wise (RBV) methods. The performance of the 2 hybrid methods, GTM+MTC and GTM+RBV were compared with that of the original MTC and RBV techniques. The data processing procedures can be outlined as follows: (1) Segmentation of 1H T1-MPRAGE images to extract tissue probability maps for CSF, grey and white matter; (2) Co-register the 23Na MRI and tissue probability maps to MNI template; (3) Apply the different PVC methods to the co-registered 23Na images using different PSF (FWHM varied 3 to 20 mm); (4) Evaluate the corrected 23Na images by estimating the actual FWHM and STC ratios.Results
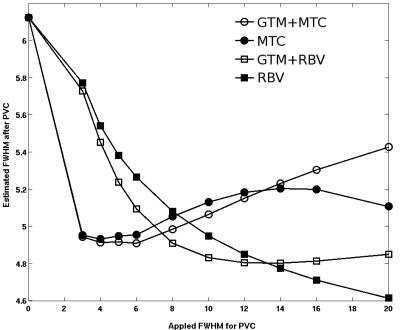
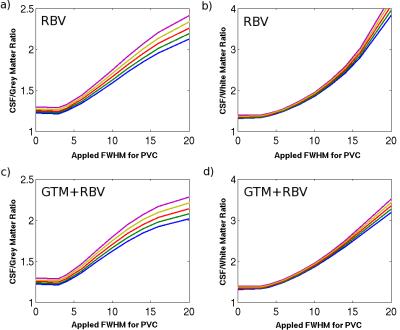
Fig. 1 shows an axial slice of the corrected 23Na images using the hybrid GTM+RBV method with different PSF parameters. With increased FWHM, the blurring effects due to T2* decay and finite sampling grid are reduced. Fig. 2 depicts the actual spatial resolution of the corrected images after applying the different PVC methods and PSF. It appears that the MTC based methods are less reliable than RBV, MTC can result in over correction when inappropriate PSF is applied. The hybrid method GTM+RBV is most robust. Fig. 3 exhibits the STC ratio results for the RBV-based corrections. As expected, with the reduced partial volume effects (PVEs) the ratios are increased approaching the values obtained with high resolution 23Na MRI at 9.4T (7).Discussion
There are two types of PVEs that degrade quantification accuracy of 23Na MRI. One is the blurring due rapid T2* relaxation during data acquisition. The other is the tissue fraction effect related to the finite spatial resolution causing multiple tissue types existing within a single voxel and each tissue type may contribute differently to the 23Na MRI signal. PVEs become very relevant for the study of neurodegenerative diseases, because they can induce apparent changes in the signal as illustrated in Fig. 3. To mitigate PVEs caused by the spatial blurring, data-driven methods based on iterative dconvolution approaches have been applied previously in PET. The tissue-fraction effect can only be addressed with the inclusion of anatomic information from 1H MRI. However, most anatomy-based PVC techniques in the PET literature rely on the assumption of regional uniformity, which will likely induce bias. The idea of hybrid approach is to extend the RBV correction by iteratively modifying region boundaries and calculating changes to regional variability based on estimates of corrected voxel values. Since T2* for Na+ in different tissues differ substantially, we need to consider the PSF variations in different brain regions, even though the performance of GTM+RBV is robust when FWHM is sufficiently large (see Fig. 2).Conclusion
In this study we have focused on the development and application of PVC techniques for improving the quantification accuracy of STC with 23Na MRI at 3T. Although PVEs are known to induce errors in quantification, it has not been widely used in 23Na MRI. While different PVC algorithms have been proposed in the PET literature, each method has its limitations and relies on simplified assumptions. Hybrid methods, such as GTM+RBV, aimed to overcome such limitations are most robust.Acknowledgements
Experimental assistance from S. Salinas is gratefully acknowledged.References
1. Atkinson IC, Lu A, Thulborn KR. Clinically constrained optimization of flexTPI acquisition parameters for the tissue sodium concentration bioscale. Magn Reson Med 2011;66(4):1089-1099.
2. Thulborn KR, Lu A, Atkinson IC, Damen F, Villano JL. Quantitative sodium MR imaging and sodium bioscales for the management of brain tumors. Neuroimaging Clin N Am 2009;19(4):615-624.
3. Lu A, Atkinson IC, Claiborne TC, Damen FC, Thulborn KR. Quantitative sodium imaging with a flexible twisted projection pulse sequence. Magn Reson Med 2010;63(6):1583-1593.
4. Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med 1998;39(5):904-911.
5. Thomas BA, Erlandsson K, Modat M, Thurfjell L, Vandenberghe R, Ourselin S, Hutton BF. The importance of appropriate partial volume correction for PET quantification in Alzheimer's disease. Eur J Nucl Med Mol Imaging 2011;38(6):1104-1119.
6. Tohka J, Reilhac A. Deconvolution-based partial volume correction in Raclopride-PET and Monte Carlo comparison to MR-based method. Neuroimage 2008;39(4):1570-1584.
7. Mirkes CC, Hoffmann J, Shajan G, Pohmann R, Scheffler K. High-resolution quantitative sodium
Figures