5062
Myelin water atlas for cervical spinal cord: A template for spinal cord pathway myelin microstructure1Physics, University of British Columbia, Vancouver, BC, Canada, 2International Collaboration on Repair Discoveries, Vancouver, BC, Canada, 3Medicine, University of British Columbia, Vancouver, BC, Canada, 4UBC Hospital, University of British Columbia, Vancouver, BC, Canada, 5Kinesiology, University of British Columbia, Vancouver, BC, Canada, 6Radiology, University of British Columbia, Vancouver, BC, Canada, 7Pathology and Laboratory Medicine, University of British Columbia, BC, Canada
Synopsis
In-vivo microstructural information of myelin in the spinal cord is desirable for studying spinal cord injury and neurodegenerative diseases. We used myelin water imaging combine with Spinal Cord Toolbox to create a standard microstructure template specific to myelin content, so-called myelin water atlas, for
Introduction
With the ongoing advancements in magnetic resonance imaging (MRI), anatomical features of the spinal cord can be resolved with high resolution1, making detailed in-vivo examination of morphological changes possible. Spinal cord injuries and several neurodegenerative diseases benefit from microstructural information to evaluate the type and severity of the injury or to determine the diagnosis and prognosis of the disease.2 To our knowledge, few MR studies3 have investigated in-vivo microstructure information of different pathways in human spinal cord and, thus, variations in microstructure is not well characterized. A major limitation with assessing spinal cord microstructure is a lack of MRI techniques that are capable of differentiating distinct spinal cord pathways quantitatively and specifically in a robust manner.
Myelin, an insulating sheath around the axons, plays a critical role in the conduction of sensory and motor signals, allowing complex motor and sensory behaviors. Myelin damage or loss can occur in spinal cord injuries or diseases, affecting the proper function of the central nervous system. Therefore, we used myelin water imaging (MWI), a validated MRI method specific to myelin4,5, to quantitatively and specifically examine the spinal cord microstructure of myelin in different pathways. MWI operates on the principle that the MR signal from myelin water, the water trapped between myelin bilayers, can be extracted from the total MR signal based on a characteristic short T2 relaxation time. The ratio of myelin water signal relative to the total signal is termed myelin water fraction (MWF). In our study, we examined healthy subjects with MWI at the cervical level. Our goal was to create a microstructural myelin template, or so-called myelin water atlas, which can differentiate pathways and serve as a reference in cervical spinal cord.
Method
MRI: 19 healthy adults (12 male / 7 female, mean age 32 years, range 20-69 years) were scanned on a 3.0T MRI system (Philips, Best, The Netherlands) with a phased array spine coil. MRI data included cervical cord myelin water imaging (3D gradient and spin echo (GRASE), 32-echo, TE/TR=10/1500ms, 8 slices, resolution= 0.6x0.6x5mm3).6 The stack was centered at the level between C2 and C3 for all scans.
Myelin Water Fraction: Voxel-wise T2 decay curve analysis was performed using in-house MATLAB software to generate MWF maps from the GRASE data. This analysis employed the extended phase graph algorithm to estimate the refocusing flip angle in each voxel as well as correcting the T2 decay curve for stimulated echoes.7 MWF was defined as the fractional signal with T2 less than 40ms.
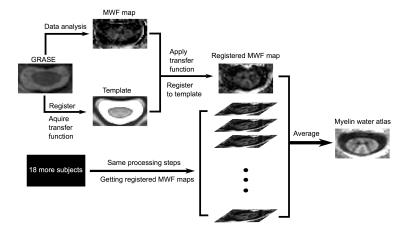
Myelin water atlas: MR images from GRASE were registered to the MNI-POLY-AMU spinal cord template using the Spinal Cord Toolbox to obtain a warp field.8 MWF maps from each subject were transformed to the spinal cord template space by applying the previously acquired warp field. The myelin water atlas was created by averaging the transformed MWF maps from all individual subjects. The workflow is depicted in Figure 1.
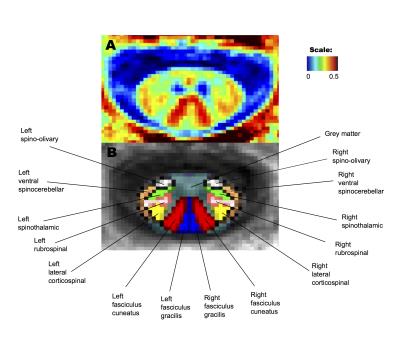
Analysis with atlas: Seven distinct bilateral pathways (dorsal columns (fasciculus gracilis and cuneatus), lateral corticospinal tract, ventral spinocerebellar tract, rubrospinal tract, spinothalamic tract, spino-olivary tract), total white matter, grey matter, and whole cord were obtained from the MNI-POLY-AMU spinal cord template and MWF values were exacted from those regions of interest (ROIs). Statistical analysis used a one-way ANOVA to test differences in all pathways and a paired t-test to examine differences in MWF between specific pathways.
Results
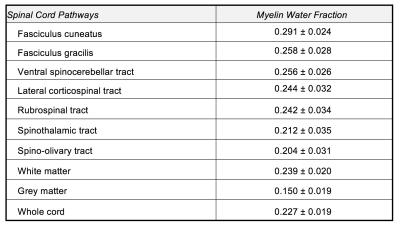
Figure 2 shows the resulting myelin water atlas and the segmentations of different pathways. Table 1 summarizes MWF in different pathways of the atlas, as well total white matter, grey matter and whole cord. One-way ANOVA showed significant differences in all pathways (F=36.289, p<0.000001). In particular, MWF was significantly higher (p<0.05, paired t-test) in the dorsal columns (0.275±0.026) compared to the spinothalamic tract (0.212±0.035). MWF was found to be highest in the fasciculus cuneatus (0.291±0.024) compared to other pathways (all p<0.00005, paired t-test) while grey matter (0.150±0.019) showed the lowest MWF as expected (all p<0.00001, paired t-test).Conclusion
The myelin water atlas shows strong agreement with well-known anatomical characteristics2, and is able to distinguish variations in myelin microstructure in 7 different spinal cord pathways. Our findings of the myelin content differences in distinct pathways agree with other MRI studies in the brainstem9,10 and histological findings in rat spinal cord11. Although more subjects and human histology validation are needed, this work shows the potential of using a myelin water atlas as a microstructure reference to visualize demyelination for other in-vivo studies of spinal cord injuries or diseases.Acknowledgements
We sincerely thank the study participants and MRI technologists at our centre. Funding support was provided by a seed grant from the International Collaboration On Repair Discoveries.References
1. Taso M, Le Troter A, Sdika M, et al. Construction of an in vivo human spinal cord atlas based on high-resolution MR images at cervical and thoracic levels: Preliminary results. MAGMA. 2014;27(3):257-267.
2. Hains DE, ed. Neuroanatomy. an atlas of structures, sections and systems 7th ed. New York: Lippincott Williams & Wilkins; 2007.
3. Duval T, McNab JA, Setsompop K, et al. In vivo mapping of human spinal cord microstructure at 300mT/m. Neuroimage. 2015;118:494-507.
4. Laule C, Leung E, Lis DK, et al. Myelin water imaging in multiple sclerosis: Quantitative correlations with histopathology. Mult Scler. 2006;12(6):747-753.
5. Laule C, Kozlowski P, Leung E, Li DK, Mackay AL, Moore GR. Myelin water imaging of multiple sclerosis at 7 T: Correlations with histopathology. Neuroimage. 2008;40(4):1575-1580.
6. Ljungberg E, Vavasour I, Tam R, et al. Rapid myelin water imaging in human cervical spinal cord. Proc. Intl. Soc. Mag. Reson. Med.
7. Prasloski T, Mädler B, Xiang Q, MacKay A, Jones C. Applications of stimulated echo correction to multicomponent T2 analysis. Magnetic Resonance in Medicine. 2012;67(6):1803-1814.
8. De Leener B, Levy S, Dupont SM, et al. SCT: Spinal cord toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2016.
9. Hong JH, Son SM, Jang SH. Identification of spinothalamic tract and its related thalamocortical fibers in human brain. Neurosci Lett. 2010;468(2):102-105.
10. Kamali A, Kramer LA, Butler IJ, Hasan KM. Diffusion tensor tractography of the somatosensory system in the human brainstem: Initial findings using high isotropic spatial resolution at 3.0 T. Eur Radiol. 2009;19(6):1480-1488.
11. Da Mata JR, Sabóia-Morais SMT, Morais JOR. Morphometric analysis of myelinated axons during maturation in the fasciculus gracilis and fasciculus cuneatus of rats. A transmission electron microscopy study. Braz.J.morphol.Sci. 2001;18(1):1-6.
Figures