5039
How to spend your time? Using multi-echo acquisition versus increasing sampling rate in resting-state fMRI1Museo Storico della Fisica e Centro Studi e Ricerche “Enrico Fermi”, Rome, Italy, 2Center for Magnetic Resonance Research, Dept. of Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
High sampling rate is pivotal for differentiating neuronal-related from spurious correlations in resting-state-fMRI (rsfMRI). Acquiring multi-echoes (ME) during an EPI readout increases contrast-to-noise, but it can compromise temporal resolution even when combined with multiband (MB). Therefore, whether MBME-EPI is ultimately beneficial for rsfMRI remains unclear. To address this, we collected data at 3T with 2-mm resolution using MBME-EPI and the human-connectome-project MB-single-echo-EPI. Data were evaluated for spectral amplitude, consistency and specificity. MBME-EPI showed significant gains in all quantities when physiological noise and sampling rate were matched between time-series. However, there was no clear gain when different sampling rates were considered.
Purpose
Multi-echo (ME) EPI can improve the blood oxygenation level dependent (BOLD) contrast-to-noise ratio (CNR)1, and can be used for removal of non-T2*-related components2. These benefits come at the expense of reduced spatial-temporal resolution compared to single-echo EPI. While the loss of temporal resolution is a bearable cost in task-based experiments, the same may not hold true in resting-state experiments, where the signal of interest is the synchronization among different brain clusters that can result from neuronal as well as spurious sources of variance. In fact, it is still poorly described whether the ME enhancement of BOLD CNR overcomes the detriments of reduced noise sampling rate in resting-state experiments. To address this, here we sought to quantify, at a given high spatial resolution, the potential gains of a multiband multi-echo (MBME)-EPI sequence as compared to a high sampling rate multiband single-echo (MBSE)-EPI sequence defined by the human-connectome-project (HCP). The gains of the optimal combination of multi-echo acquisitions were quantified in terms of spectral amplitude, consistency and specificity of BOLD low-frequency fluctuations, without specifically focusing on the impact of ME-based denoising.Methods
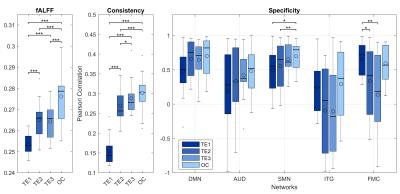
Data were collected from twelve healthy subjects, 6 young (1M/5F, 29.7±4.4 y.o.) and 6 older adults (4M/2F, 73.5±8.0 y.o.). Each subject underwent two eye-open resting-state scans with two different sequences acquired on a 3T Siemens Prisma: (1) MBSE-EPI (TE=37 ms; TR=0.77 s; MB=8; N=780) (2) MBME-EPI with 3 echoes (TEs=14.60, 37.09, 59.65 ms; TR=1.65 s; MB=4; pf=6/8; N=364). Both sequences were 10-minute long and had 2x2x2 mm3 spatial resolution. Volumes were preprocessed according to HCP minimal preprocessing pipeline3. For MBME sequence, an optimal combination (OC) of echoes was computed based on the T2* of each voxel1. Further noise removal steps were applied to each time series (i.e., each single TEs and OC), including regression of estimated realignment parameters, aCompCor correction4 and a band-pass filter (0.009-0.08 Hz). Each time-series was evaluated in terms of fractional amplitude of low-frequency (0.009-0.08 Hz) fluctuations (fALFF)5, consistency, and specificity. fALFF was calculated as aggregate values from the entire cortex. Consistency was assessed comparing how similar connectivity matrices are across the young subjects only. For this purpose, we parceled the brain in 264 ROIs6 and computed the associated correlation matrix, then, for each pair of young subjects we calculated the correlation coefficient for the two matrices. Finally, specificity was calculated from networks defined in FIG1, according to the equation7:
$$S = \frac{|z-target|-|z-reference|}{|z-target|+|z-reference|}$$
where the z-fisher transformed correlation of two regions inside a network (e.g., z-target: PCC vs MPFC), is compared against a region to whom no functional connectivity is expected (e.g., z-reference: PCC vs a visual area). An ROI in the visual system was used as a reference. Intra-sequence and inter-sequence comparisons were performed with paired t-tests. For specificity only, the non-parametric Wilcoxon test was chosen.
Results
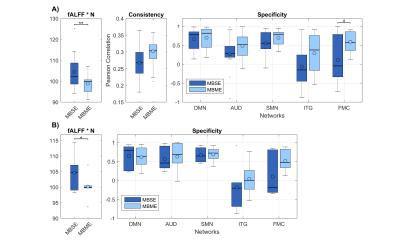
To allow a perfect match between time series in terms of physiological noise, we first considered intra-sequence comparisons within the MBME acquisition, where the OC was evaluated against each of the echoes (FIG2). Using the TE2 as a reference, significant gain in fALFF and consistency were reported. Specificity showed clear trends of increases in all considered networks and was found to be significant in the SMN. The inter-sequence comparisons are shown in FIG3A. No significant gain of MBME-OC was observed when compared to MBSE-EPI in terms of consistency. On the contrary, a significant slight decrement of fALFF was noticed. There was a gain in specificity, which however was significant only for the ITG. Analogous results were obtained selecting only subjects (n=6) whose estimated head movements were similar in the MBME-EPI and MBSE-EPI acquisitions (|Δrms| < 0.03mm; FIG3B).Discussion
The acquisition of multiple echoes, and their combination which accounts for T2* brain variability, increased the BOLD CNR leading to a significant gain in all considered resting-state parameters. However, the gain was fully realized only when comparing time series with the same physiological noise and sampling rate. When the MBME-OC was compared with the higher temporal resolution MBSE-EPI, the advantage was partially lost, indicating that resting-state experiments do not benefit from increased CNR more than from increased noise discrimination power. Further studies are needed to evaluate the impact of ME-based removal of non-T2*-related components and/or other denoising pipelines such as FIX8, recently proven to be particularly beneficial for multiband approaches.Conclusion
With the present MBME-EPI sequence and with a common denoising pipeline, the gain in BOLD CNR due to the OC of different echo times does not overcome the detriment of temporal resolution in resting-state data.Acknowledgements
This work was supported by U01AG052564, P41 EB015894, P30 NS076408. This project has also received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 691110 (MICROBRADAM).References
1. Posse S, Wiese S, Gembris D, et al. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magnetic resonance in medicine.1999;42(1):87-97.
2. Kundu P, Inati SJ, Evans JW, et al. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage. 2012;60(3):1759-1770.
3. Glasser MF, Sotiropoulos SN, Wilson JA, et al. "The minimal preprocessing pipelines for the Human Connectome Project." Neuroimage. 2013;80:105-124.
4. Behzadi Y, Restom K, Liau J, et al. "A component based noise correction method (CompCor) for BOLD and perfusion based fMRI." Neuroimage. 2007;37(1):90-101.
5. Zou QH, Zhu CZ, Yang Y, et al. "An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF." Journal of neuroscience methods. 2008;172(1):137-141.
6. Power JD, Cohen AL, Nelson SM, et al. "Functional network organization of the human brain." Neuron. 2011;72(4):665-678.
7. Weissenbacher A, Kasess C, Gerstl F, et al. "Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies." Neuroimage. 2009;47(4):1408-1416.
8. Griffanti L, Salimi-Khorshidi G, Beckmann CF, et al. "ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging." Neuroimage. 2014;95:232-247.
Figures