5037
Echo-planar imaging with the Dynamic Multi-Coil Technique (DYNAMITE-EPI)1Yale School of Medicine, New Haven, CT, United States, 2RWTH Aachen University, Aachen, Germany
Synopsis
Despite numerous improvements in MRI technology, the fundamental gradient hardware has always been designed to generate linear and orthogonal fields. This mould was broken with the advent of DYNAmic Multi-coiIl TEchnique (DYNAMITE), which uses non-linear and non-orthogonal B$$$_{0}$$$ fields for shimming and imaging. Besides vastly expanding the field shaping possibilities, this new technology also promises faster switching and lower eddy currents. For widespread uptake of this technology, proving its suitability for performing widely used contemporary scans like EPI is a must. In this work we share our results from the first successful implementation of DYNAMITE-EPI.
Purpose
In recent years hardware to produce non-linear and non-orthogonal B$$$_{0}$$$ fields, termed Multi-coil has been introduced [1]. Based on this hardware, the DYNAmic MulticoIl TEchnique(DYNAMITE) has been successfully used for B$$$_{0}$$$ shimming [2–4] and imaging [5, 6]. For DYNAMITE to become a mainstream imaging technique, one has to demonstrate its ability to perform fast imaging acquisitions like echo-planar imaging (EPI), which is ubiquitous in most MRI studies. This work reports the first successful implementation of multi-slice EPI using DYNAMITE, without using the scanners’ built-in conventional gradient system. This development further cements the role of DYNAMITE as a viable imaging technique.Methods
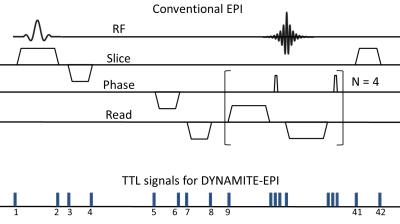
Experiments were conducted on a 9.4 T pre-clinical scanner. Previously described Multi-coil hardware [6] comprising 48 coils was used for this study. Identical gradient-echo (GE) EPI sequences were designed, with conventional linear gradients and utilizing Multi-coil hardware only. The acquisition parameters were fully matched to make a one-on-one comparison between EPI images obtained from the two coil setups. For DYNAMITE-EPI, the same set of coils was used to produce all the necessary gradients (X, Y, Z). The magnetic field modeling was based on least-squares fitting as described earlier [6]. The calibration scans for DYNAMITE current estimation were obtained from an asymmetric-spin echo B$$$_{0}$$$ mapping sequence [7]. This allowed the usage of 90% coil current dynamic range, leading up to 6 kHz B$$$_{0}$$$ deviation measurements for calibration. The multi-slice EPI scan parameters were: FOV of 12x12 mm$$$^{2}$$$, 11 slices (1 mm thick), 64x64 in-plane resolution, echo time 16 ms, repetition time 1 second. An acquisition bandwidth of 36 kHz was chosen based on the available $$$\pm$$$ 1 A dynamic amplifier range per coil. An 8-shot EPI acquisition scheme was adopted to keep the readout duration reasonably short (18 ms) and to minimize the susceptibility to B$$$_{0}$$$ inhomogeneity. EPI acquisitions were performed on a phantom object and a carrot. Identical non-linear phase correction was used to reconstruct the EPI images. For DYNAMITE-EPI, appropriate coil currents to generate the required gradient patterns were set in real time using a series of TTL pulses [6]. For each shot of EPI acquisition, 42 events comprising current settings for all the 48 coils were employed (see Fig. 1). Overall, the DYNAMITE-EPI sequence consisted of 42x11(slices)x8(shots)=3696 events. The setting of coil currents within and across the shots was fully automated, thereby enabling interaction free scanning.Results
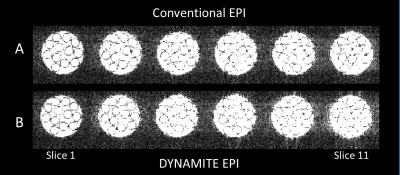
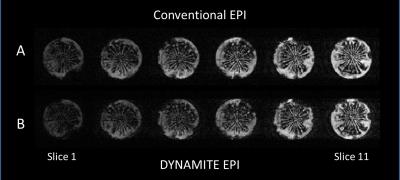
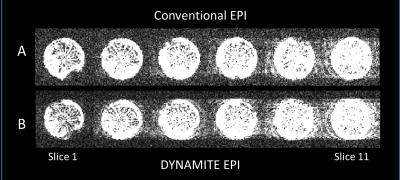
We observed near identical slice selection profiles with both coil setups in our experiments, similar to our previous work [6]. DYNAMITE and conventional EPI acquisitions produced nearly identical images (Fig. 2A,B), and the ghosting artefacts were minimal in both sequences (Fig. 3A,B). The similarity of images across slices and in-plane show that the linear fields produced by DYNAMITE were accurate across the object in all three spatial directions. The absence of artefacts beyond those apparent with conventional EPI confirms both fidelity and accuracy of the switching behavior of DYNAMITE hardware during the EPI sequence, and, moreover, the multi-echo readout train. Similar EPI results from a carrot are shown in Figs.4 and 5.Discussion
EPI is the workhorse behind some of the most important applications of MRI, like functional MRI and diffusion imaging. Combined with our previous success in using DYNAMITE for radial imaging [6], this demonstration of DYNAMITE-EPI is a significant milestone in establishing the suitability of DYNAMITE for MRI. While linear fields are a small subset of the versatile field-shaping capability of Multi-coil hardware, this work demonstrates its excellent switching capabilities. Notably, the experiments reported here aimed to match the DYNAMITE EPI sequence to the conventional EPI implementation. Due to the very short rise time of Multi-coil hardware (10 $$$\mu$$$s), gradient switching for DYNAMITE-EPI can be performed at this time scale and allows very short echo times or longer echo trains. Moreover, since Multi-coil hardware does not touch the magnet bore, the induced eddy-currents are negligible. These two factors will benefit DYNAMITE EPI significantly. The limited dynamic range of our current Multi-coil hardware poses some constraint for the realization of larger gradient amplitudes. However, the application of stronger amplifiers is straight forward and expected to significantly increase the acquisition bandwidth, thereby paving the way for shorter readout times and fewer or single shot EPI. Notably, larger dynamic range will also allow to merge readout and phase encoding events that are played separately in this DYNAMITE-EPI implementation. Barring these minor achievable improvements, our current results clearly demonstrate the potential of fast imaging with DYNAMITE-EPI.Acknowledgements
No acknowledgement found.References
1. Juchem C, Nixon TW, McIntyre S, Rothman DL, and de Graaf RA. Magnetic field modeling with a set of individual localized coils. Journal of Magnetic Resonance, 2010, 204(2):281 – 289.
2. Juchem C, Brown PB, Nixon TW, McIntyre S, Rothman DL, and de Graaf RA. Multicoil shimming of the mouse brain. Magnetic Resonance in Medicine, 2011, 66(3):893–900.
3. Juchem C, Nixon TW, McIntyre S, Boer VO, Rothman DL, and de Graaf RA. Dynamic multi-coil shimming of the human brain at 7T. J Magn Reson, 2011, 212:280 – 288.
4. Juchem C, Rudrapatna SU, Nixon TW, and de Graaf RA. Dynamic multi-coil technique (DYNAMITE) shimming for echo-planar imaging of the human brain at 7 Tesla. NeuroImage, 2015, 105:462– 472.
5. Juchem C, Nixon TW, and de Graaf RA. Multi-coil imaging with algebraic reconstruction. ISMRM, 2012, (2545).
6. Juchem C, Nahhass OM, Nixon TW, and de Graaf RA. Multi-slice MRI with the dynamic multi-coil technique. NMR in Biomedicine, 2015, 28(11):1526–1534.
7. Bartusek K, Dokoupil Z, and Gescheidtova E. Magnetic field mapping around metal implants using an asymmetric spin-echo MRI sequence. Measurement Science and Technology, 2006, 17(12):3293.
Figures