5023
Model-enhanced ZTE rendering of musculoskeletal structures1GE Healthcare, Cambridge, United Kingdom, 2GE Healthcare, United States
Synopsis
The objective of the present study was to improve three-dimensional visualisation of ZTE-based bone images by incorporating a-priori anatomical information in the rendering process.
Purpose
Volume rendering of musculoskeletal zero echo time (ZTE) datasets has been recently proposed for clinical diagnosis and therapy planning. Indeed, short echo time sequences are known to capture the fast-decaying signal from bone tissue (T2 ~ 0.39 ms at 3T)1 and proton-density weighted ZTE data have been shown to correlate with computed tomography (CT) measurements of bone density2.The potential of three-dimensional visualisation of musculoskeletal structures without the radiation dose associated with CT is evident.
However, there are several obstacles to the generation of usable renderings: Firstly, ZTE data relies on the identification of the cortical layers of bone, which can in some areas be thinner than the acquisition resolution and therefore become severely affected by partial volume. Secondly, there are unwanted anatomical structures that display similar properties to cortical bone in the ZTE images, especially when the definition of cortical bone has to be relaxed to account for partial volume effects.
The result is a tradeoff between the detail with which bone can be depicted and the amount of undesired structures that will appear in the rendering. Due to the nature of image rendering, these structures will eclipse and obscure some view angles, potentially hampering diagnosis. The objective of the present study was to overcome this tradeoff by incorporating a-priori anatomical information in the rendering process.
Methods
A new model-enhanced rendering method was developed and tested on a set of ten ZTE acquisitions of healthy subjects. Knee acquisitions were chosen for this first study due to the favorable morphology and limited range of flexion allowed by the local coil.
The acquisitions were performed on a GE Discovery MRI scanner using an acquisition protocol similar to that described by Breighner et al3. Small, isotropic pixel sizes (~1.4mm) are desirable to minimize partial volume, whereas low flip angles (~1deg) are required to prevent T1 saturation, which would increase the number of false-positive bone structures. This, together with the relatively high bandwidth (±62.5kHz) needed to capture short-T2 species, leads to very low SNR requiring multiple averages (NEX 4-6).
Prior to rendering, bias correction, non-patient object removal and intensity remapping were performed using a custom C++ algorithm. The Insight Segmentation and Registration Toolkit (ITK) was used for this purpose. A knee model based on CT data was then registered to the remapped ZTE data. The model fitting procedure consisted on a rigid initialization, followed by affine and then non-rigid B-Spline registration. Thanks to the remapping of ZTE intensities to pseudo-CT values2, it was possible to use an efficient sum-of-squares similarity criterion.
Rendered views of the resulting bone-enhanced dataset were generated using the Visualization Toolkit (VTK) without operator intervention. A signed distance map to the registered model was used to control the smoothing, enhancement and visibility of the voxels.
Results
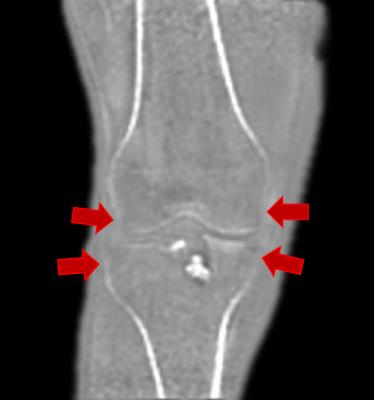
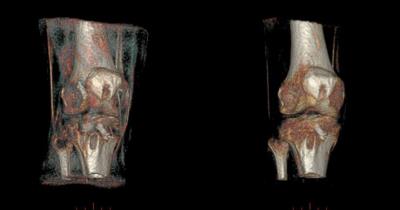
As previously reported, proton density weighted ZTE acquisitions successfully captured the signal from the cortical layer of bone. The remapping of these datasets to pseudo-CT density estimates could be consistently achieved by an automated procedure for the selected range of acquisition settings. Automated rendering of the remapped volume was also successful, but presented several limitations: In all cases, the density values of thinner cortical regions of the femoral, tibial and fibular epiphyses were underestimated due to partial volume, as illustrated in Figure 1. Furthermore, a number of ligaments (e.g. patellar, quadriceps, collateral) and tendons (e.g. hamstrings) appeared as high-opacity structures in the rendering. While the former may be of clinical value, the latter are just obscuring the view of the joint.
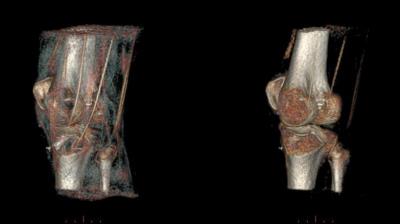
Both of the above limitations could be mitigated with the model-enhanced rendering. Non-rigid model registration could effectively overcome inter-individual morphological disparity, within ±2 voxels on average. The inspection of reformatted views of the processed results confirmed that model structures were not being incorporated to the images (i.e. the relevant anatomy displayed remained that of the original measurement). Figures 2 and 3 illustrate the results without and with a priori anatomical information.
Discussion
By non-rigidly registering an anatomical model of the region of interest, it becomes possible to apply more sophisticated processing to restore cortical regions affected by partial volume, which was previously unsuccessful due to the similarity of these regions –in terms of low-level image features– with a number of unwanted anatomical structures (e.g. chemical shift on the interfaces of large soft-tissue structures, like muscle packages). Structures that would degrade the rendering (e.g. tendons, low-SNR areas, saturated tissue) can be selectively smoothed or attenuated.
The key enabler for this method is the ability to generate pseudo-CT density estimates based on ZTE data, which greatly simplifies the model registration problem. Conversely, once overcome the initial challenge of registration, a-priori anatomical information can facilitate some of the otherwise complex image processing steps required to generate pseudo-CT maps (e.g. bias correction and tissue masking). A two-stage approach is currently under investigation which could potentially relax some of the constraints on the acquisition parameters, providing more accurate depiction of short T2 structures.
Conclusion
With the incorporation of a-priori anatomical information, it is possible to mitigate the tradeoff between bone and artifact rendering. Dedicated enhancement algorithms can be applied exclusively to the bone, improving the visibility of the thinner cortical regions, while non-bone regions can be processed to minimize the visibility of unwanted structures.Acknowledgements
No acknowledgement found.References
1 Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J Magn Reson. 2010 Dec;207(2):304-11.
2. Wiesinger F, Sacolick LI, Menini A, Kaushik SS, Ahn S, Veit-Haibach P, Delso G, Shanbhag DD. Zero TE MR bone imaging in the head. Magn Reson Med. 2016 Jan;75(1):107-14.
3. Breighner R, Eagle S, Delso G, Potter HG, Koff MF. ZTE Imaging of Joints: Unmasking the Bone. ISMRM 2016; 2274.Figures