5013
Highly Efficient Bi-Component T2* Mapping of the Knee using Ultra-short Echo Ramped Hybrid Encoding1Department of Radiology, University of Wisconsin, Madison, WI, United States
Synopsis
T2* analysis is used in musculoskeletal imaging to characterize tendon, meniscus, and cartilage in human joints. With the development of high performance gradient systems, ultrashort time echo (UTE) imaging has become more feasible, allowing robust bi-component of short and long T2* tissue components. Many studies have been performed to realize robust and clinically feasible bi-component T2* imaging, but the long acquisition time required to obtain multiple echo images remains challenging. In this study, we propose a novel, rapid imaging scheme for bi-component T2* analysis, based on ramped hybrid encoding (RHE) that allows robust bi-component T2* estimation within a single scan.
Purpose
Ultrashort time echo (UTE) T2* mapping techniques have been used to evaluate cartilage, tendon, meniscus, ligament, and cortical bone1-4. The use of bi-component imaging and reconstruction methods can improve the specificity of T2* analysis of musculoskeletal tissues with multiple water components. However, long acquisition times, limited anatomic coverage, and image artifacts associated with current techniques have reduced the feasibility of bi-component T2* analysis in clinical studies. In this study, we propose a novel UTE imaging method based on ramped hybrid encoding (RHE)5 to provide rapid bi-component T2* analysis of the human knee joint at 3T.Methods
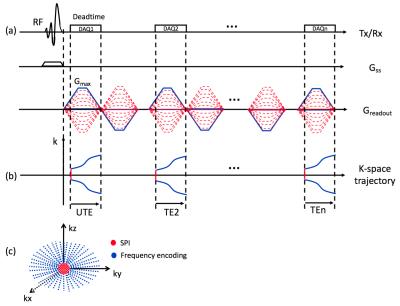
Image Strategy: Multi-echo RHE acquires a UTE image followed by gradient-echo images in later echo times (TEs) as shown in Figure 1-a, where central region of k-space is acquired by Cartesian SPI (red lines in Figure 1-b), and outer k-space is acquired by radial frequency encoding (blue lines in Figure 1-b). In RHE, gradients are turned on before the RF coil deadtime and ramped up to maximum gradient, Gmax, to minimize readout duration. After the UTE acquisition, a flying-back gradient echo train is applied to acquire later echoes. To enable slab selectivity, a SLR half-pulse was used. k-Space trajectory was measured using a SPI-based gradient measurement technique6, which is crucial in radial gradient-echo imaging with long echo train length due to accumulated errors in trajectory, causing severe imaging artifact if reconstructed with nominal k-space trajectory.
Image Acquisition and Analysis: Multi-echo RHE was performed on the knee of one healthy volunteer using a 3T scanner (MR750, GE Healthcare, Waukesha, WI) and 8-channel T/R extremity coil with the following imaging parameters: Gmax=50mT/m, slewrate=200mT/m/ms, readout duration=600µs, spatial resolution=0.6x0.6x3mm3, FOV=18x18x3cm3 or 15x15x15cm3, TR=33ms, TE=0.08 2.0 3.9 5.8 7.7 9.6 11.5 13.4 15.3 17.1 19.0 20.9 22.8 24.7 26.6 28.5 30.4ms, and 8 minute scan time. SPI was encoded using 15 phase encoding steps in one axis, where a 3D spherical region in central k-space is covered. 9180 radial spokes were used for frequency encoding to cover k-space with an ellipsoidal shape with reduced coverage in the z-axis (L-R) as shown in Figure 1-c. A fat saturation pulses was applied every TR before the RHE acquisition to suppress fat signal. Images were reconstructed using convolution gridding with oversampling rate=1.5 and kernel width=3 pixel, using the measured k-space trajectories. For comparison, a 3D-Cones UTE acquisition with 16 echoes and 10 slices through the patellar tendon and 12 minute scan time (cumulative time for four separate scans) was performed on the knee of the same subject3,7. Mono-component and bi-component exponential signal models implementing a non-linear least square fitting method under the assumption of a Rician-distributed noise8 were used to characterize the water components in musculoskeletal tissues.
Results
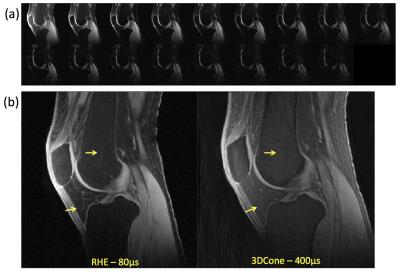
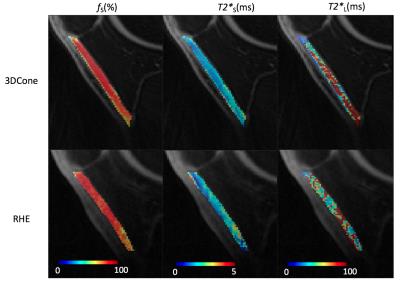
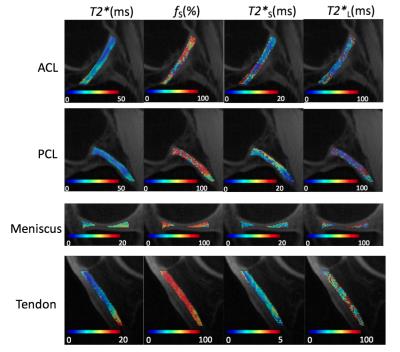
Figure 2-a shows sagittal images through the knee at 17 echoes acquired using RHE. There was a monotonic decay of the signal for all tissues with much stronger decay occurring prior to 2ms for patellar tendon. Figure 2-b compares the UTE echo image acquired by RHE and 3D-Cones, where RHE shows better resolution, less artifact, and sharper tissue boundaries owing to the shorter readout duration in RHE (600µs) compared to 3D-Cones (1.2ms). As shown in Figure 3, RHE provides similar parameter estimations of the patellar tendon with good fitting quality similar to 3D-Cones. Figure 4 shows bi-component T2* maps of ligament, meniscus, and tendon of the knee acquired using RHE which provide similar parameter estimations as previously published techniques2,3.Discussion and Conclusion
This study has demonstrated the feasibility of a single acquisition multi-echo RHE imaging method to provide rapid and reliable bi-component UTE T2* analysis of ligament, meniscus, and tendon within the human knee joint at 3T. The current 8 minute scan time of RHE could be further reduced by optimizing fat saturation, which takes 23% of the total imaging time. In contrast to other acquisition techniques, the later echoes in multi-echo RHE are more robust to B0 inhomogeneity, magnetic susceptibility artifact, and eddy current induced trajectory distortion due to the benefits of SPI9. With a single scan strategy, all echoes needed to perform bi-component signal fitting are acquired at a similar time making the method less sensitive to model fitting errors due to patient motion artifact. The current RHE sequence can provide rapid bi-component T2* analysis of ligament, meniscus, and tendon. Further technical development is necessary to allow the acquisition of later echoes using the RHE method to perform bi-component T2* analysis of cartilage.Acknowledgements
We acknowledge support from NIH EB013770, NIH AR068373-01, GE Healthcare, and University of Wisconsin Department of Radiology Research and Development Committee.References
1. Li S, Chang EY, Bae WC, Chung CB, Gao S, Bao S, Bydder GM, Hua Y, Du J. Ultrashort echo time bi-component analysis of cortical bone--a field dependence study. Magn Reson Med 2014;71(3):1075-1081.
2. Juras V, Apprich S, Zbýn S, Zak L, Deligianni X, Szomolanyi P, Bieri O, Trattnig S. Quantitative MRI analysis of menisci using biexponential T2 * fitting with a variable echo time sequence. Magn Reson Med 2013.
3. Chang EY, Du J, Iwasaki K, Biswas R, Statum S, He Q, Bae WC, Chung CB. Single- and Bi-component T2* analysis of tendon before and during tensile loading, using UTE sequences. J Magn Reson Imaging 2015;42(1):114-120.
4. Du J, Diaz E, Carl M, Bae W, Chung CB, Bydder GM. Ultrashort echo time imaging with bicomponent analysis. Magn Reson Med 2012;67(3):645-649.
5. Jang H, Wiens CN, McMillan AB. Ramped hybrid encoding for improved ultrashort echo time imaging. Magn. Reson. Med. 2016;76:814–825. doi: 10.1002/mrm.25977.
6. Jang H, McMillan AB. A rapid and robust gradient measurement technique using dynamic single-point imaging. Magn. Reson. Med. 2016;00. doi: 10.1002/mrm.26481.
7. Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med 2006;55(3):575-582.
8. Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magn Reson Med 1995;34(6):910-914.
9. Wiens CN, Artz NS, Jang H, McMillan AB, Reeder SB. Externally calibrated parallel imaging for 3D multispectral imaging near metallic implants using broadband ultrashort echo time imaging. Magn. Reson. Med. 2016;00:1–7. doi: 10.1002/mrm.26327.
Figures