5009
MUSCLE: MUlti SliCe Localized Excitation 31P-MRS1Medical Physics Group, Institute of Diagnostic and Interventional Radiology, Jena University Hospital - Friedrich Schiller University Jena, Jena, Germany
Synopsis
We present a new 31P-MR spectroscopy pulse sequence, the so called Multi-SliCe-Localized-Excitation approach (MUSCLE), which enables time resolved, interleaved non-spin-echo acquisitions of spectra in multiple muscle slabs. The accuracy of slab selection was successfully verified at 3 T by in vitro measurements in a multiple compartment phantom as well as by in vivo measurements of moderately loaded human calf muscles.
Purpose
Phosphorous MR spectroscopy (31P-MRS) allows direct insight into muscle metabolism as it enables quantitation of load induced changes of high energy compounds, like phosphocreatine (PCr), inorganic phosphate (Pi) as well as pH values1. High temporal resolution and spatial localization are crucial to detect rapid metabolic changes within heterogeneously loaded muscle groups. However, most of the available 31P-MRS techniques, for example fast FID sequences with coarse signal localization via the sensitivity profile of a surface RF coil or Chemical Shift Imaging (CSI), miss to address both of these requirements. Consequently, more appropriate methods, for example the DRESS technique2 or multi-voxel localization by a semi-LASER approach3 have been introduced recently. While the former method provides fast measurements only in single volumes, the latter technique suffers from the undesirable SNR decrease due to prolonged echo times (TE). Therefore, in this work, we introduce an alternative 31P-MRS method with MUlti-SliCe-Localized-Excitation (MUSCLE), which combines the advantages of the mentioned techniques. We evaluated this method by means of measurements in a multiple compartment phantom as well as in human calf muscles.Materials and Methods
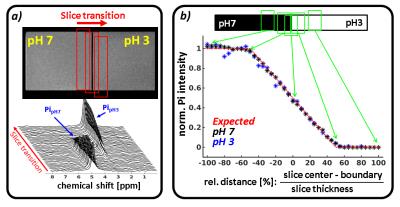
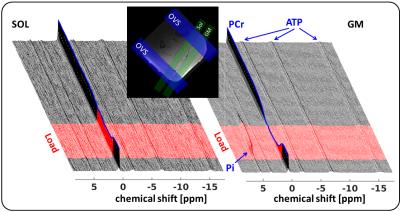
The implemented MUSCLE sequence (Siemens IDEA, VE11B) combines slice selective excitation with fast spectroscopic FID data sampling. It encompasses successive iterations (Nit) each containing a series of excitations of spatially shifted slices, Nsl, whose positions, profiles and thicknesses can be adjusted by rf-pulse frequency, rf-pulse duration and slice gradient amplitude, respectively (Fig. 1). Up to eight localized, outer volume suppression bands with variable thickness can be selected to suppress the inner-slice magnetization in adjacent muscles and improve the selection of the volume-of-interest. The accuracy of the spatial selection was evaluated in vitro by a series of single 31P-MR measurements (TR: 5 s, Nit: 5, TE: 1.2 ms, 1.6 ms rf pulse) in 20 mm thick slices transiting between two phantom chambers with pH-7 and pH-3 Pi solutions (Fig. 2). In addition, time series of pre-load (1.5 min), load (3 min) and post-load (11 min) spectra (TR: 5 s, TE: 1.2 ms, 1.6 ms rf pulse, Fig. 3) were acquired in two 15 mm thick slices positioned in the right m. gastrocnemius medialis (GM) and m. soleus (SOL) of six healthy males (24 -25 years), who exercised moderately by performing plantar flexion in a MR compatible pedal ergometer4 (48 angulation per minute, 0.5 bar pedal resistance). All measurements were conducted in a clinical whole body 3 T MR scanner (Prisma Fit VE11B, Siemens Healthineers AG) by using a flexible, double tuned surface coil (1H/31P, Ø: 11 cm, RAPID-Biomedical). Metabolic Pi and PCr intensities were quantified with the AMARES tool of the jMRUI 4.0 package (http://www.jmrui.eu). Inter-muscular pH values were determined from the Pi chemical shifts1.Results
Fig. 2b demonstrates the good agreement (max error: 4 %) between the measured evolutions of pH-7 (black asterisks) as well as pH-3 (blue asterisks) Pi intensities and the initial intensity course (red dashed line), which is expected by the slice transition between the phantom chambers. Fig. 3 shows representative series of in vivo 31P-MR spectra measured with the MUSCLE sequence in SOL (left) and GM (right) prior, during and after calf muscle exercise. Consistent with comparable previous studies2,3 on localized muscle measurements, our spectra series did not reveal any obvious Pi peak splits, which typically occur as a consequence of spatial contaminations from adjacent tissue. As expected, higher loaded GM revealed distinctly larger PCr drops and lower end exercise pH values (PCr drop: 68 ±20 %; end exercise pH: 6.78 ±0.18) than the moderately activated SOL muscle (PCr drop: 42±1 %; end exercise pH: 6.93±0.08).Discussion and Conclusion
In our current work, we have implemented a simple sequence allowing interleaved excite-acquire 31P-MRS measurements in multiple tissue regions without using longer echo times and no T2 relaxation related signal attenuations. Our approach provides high localization accuracy as demonstrated by an excellent matching of Pi intensity evolutions, which were sampled through the slice transition in a multi-compartment Pi phantom with Pi signal courses as initially expected. The high spatial selectivity was also reflected by disappearing Pi splits in in vivo spectra from loaded calf muscles as well as by distinctly different pH and PCr adaptations in heterogeneously load muscles groups.Acknowledgements
References
1. Kemp, G.J. Muscle Studies by 31P MRS. eMagRes. 2015; 4(1): 525-534.
2. Valkovic, L. et al. Depth-resolved surface coil MRS (DRESS)-localized dynamic (31) P-MRS of the exercising human gastrocnemius muscle at 7 T. NMR Biomed. 2016; 29(1): 57-65
3. Niess, F. et al. Interleaved multivoxel 31P-MR spectroscopy. Magn Reson Med. 2016, published online
4. Tschiesche, K. et al.MR-compatible pedal ergometer for reproducible exercising of the human calf muscle. Med Eng Phys. 2014; 36(7):933-7.
Figures