5006
Crossing muscle fibres in the tongue resolved using constrained spherical deconvolution1Department of Head and Neck Oncology and Surgery, Netherlands Cancer Institute, Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands, 2Department of Radiology, Academic Medical Center, Amsterdam, Netherlands, 3Department of Oral and Maxillofacial Surgery, Academic Centre for Dentistry Amsterdam and Academic Medical Center, University of Amsterdam and VU University Amsterdam, Amsterdam, Netherlands, 4Biomedical NMR, Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 5Orthopaedic Research Lab, Radboud UMCN, Nijmegen, Netherlands, 6Department of Oral and Maxillofacial Surgery, Academic Medical Center, Amsterdam, Netherlands, 7Department of Robotics and Mechatronics, MIRA Institute, University of Twente, Enschede, Netherlands, 8Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 9Biomedical Engineering and Physics, Academic Medical Center, Amsterdam, Netherlands
Synopsis
Tongue muscle architecture is suspected to be important in the prediction of speech and swallowing complications after surgery. The tongue contains areas of crossing muscle fibres unable to be resolved by diffusion tensor imaging (DTI). We show that constrained spherical deconvolution (CSD) is able to distinguish these crossing fibres ex vivo and in vivo using a clinically acceptable scan time of 10 min. Also, we show improved tractography in CSD compared to DTI, allowing segmentation of different tongue muscles which conforms to known anatomy.
Purpose
For patients suffering from cancer of the oral cavity or oropharynx, the choice for surgical removal is based on post-operative complicated swallowing and speech, which is difficult to predict.1 Post-operative functionality of the tongue is suspected to depend on muscle architecture. A method for imaging muscle architecture in vivo, is diffusion tensor imaging (DTI).2 However, contrary to most skeletal muscles, the tongue contains areas of crossing fibres, primarily in the tongue core, which hinder correct anatomical reconstructions with DTI. Therefore, we propose to use constrained spherical deconvolution (CSD)3 to resolve crossing fibres in the tongue in clinically acceptable scan time.Methods
One ex vivo bovine tongue and five healthy volunteers were scanned using a 3T Philips Ingenia MRI-scanner (Philips Healthcare, Best, Netherlands). The differences in acquisition protocol between the ex vivo and in vivo subjects were kept to a minimum.
Ex vivo: One bovine tongue was scanned using a conventional torso coil within 24h after harvesting. Scanning parameters were: spin-echo-single shot EPI; ETL 25; TE/TR 60ms/25s; two repetitions with opposing phase-encoding; NSA = 1; SPIR and SSGR fat suppression; field-of-view of 192x156x420 mm; voxel size 3x3x3mm; b-value 700 s/mm2, along 64 directions evenly spaced over a hemisphere and optimised for gradient load.
In vivo: Five healthy volunteers scanned supine using two flexible surface coils (diameter 15 cm). The coils were gently strapped to the cheeks. Subjects were instructed to position the dorsum of the tongue against the palate to minimise air in the oral cavity. Scanning parameters were: spin-echo single-shot EPI; TE/TR 60ms/3.4s; field-of-view 192x156x84 mm. The acquisition was divided into four blocks of 2.5 min, minimising motion during the scan, resulting in a total scan time of 10 min. The acquisition was repeated within 30 min. The other parameters were identical to the ex vivo acquisition.
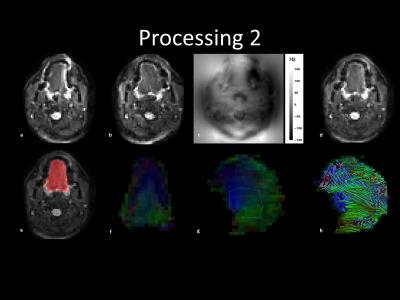
Processing: B0-field inhomogeneity and eddy current correction were performed in FSL’s Topup algrithm4,5 using images of opposite phase-encoding (figure 1a-d). The images were denoised in DTITools for Mathematica6 based on a Rician distribution. Tongue masks were created by manually segmentation (figure 1e). Images with motion artefacts were removed if the mean intensity within the mask was more than twice the standard deviation lower than the mean. In ExploreDTI7, tensors were estimated using RESTORE8. DTI tractography of the in vivo genioglossus was performed using the settings: FA range 0.1-0.6; 3mm seed point resolution; 1 mm step size; 15° angular threshold; tract length range 10-100mm. The reproducibility of FA and MD was determined using the within-subject coefficient of variation (CoV). CSD was calibrated by manually selecting an ROI in the genioglossus (figure 1g) (settings: Lmax 8; peak threshold 0.3 ex vivo and 0.1 in vivo). Finally, CSD tractography was performed with the same parameters as DTI tractography except the FA constraint (figure 1h).
Results
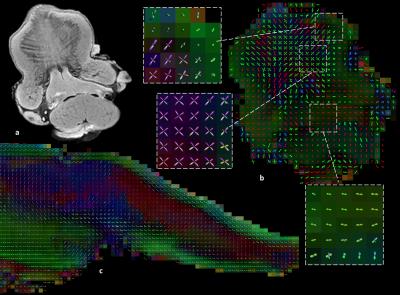
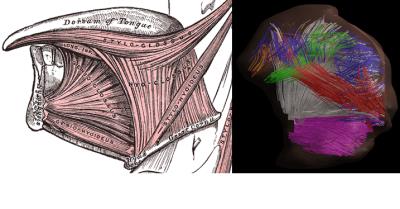
Mean FA and MD values for DTI of the in vivo genioglossus were 0.24 and 1.47·10-3 mm2/s, with a CoV of 3.5% and 2.1% for FA and MD respectively. CSD displays the ability to resolve multiple fibre directions in ex vivo bovine tongue muscles (figure 2). Figure 3 shows the improvement in resolving crossing fibres using tractography in vivo for CSD compared to DTI. In whole volume CSD tractography, various tongue muscles can be segmented, which conform to known anatomy (figure 4).Discussion
Despite severe image distortions due to B0-field inhomogeneities, good quality images could be reconstructed using Topup, strengthened by the good reproducibility of both FA and MD in the in vivo genioglossus. However, FA and MD values displayed were slightly lower than reported in literature2, which may be explained by the use of higher b-values in this study. The ex vivo CSD glyphs appear to be more sensitive to noise compared to in vivo situation, which may be explained by lower MD observed in ex vivo tissue. We show that CSD of the tongue can resolve crossing fibre populations ex vivo and in vivo, similarly to other advanced diffusion techniques9,10. Accurate reconstruction of these crossing tongue core muscle fibres may prove to be important for the prediction of speech and swallowing loss after surgery.Conclusion
CSD shows improved resolving of crossing fibres in the tongue compared to DTI using a clinically acceptable scan time of 10 min.Acknowledgements
No acknowledgement found.References
1. Kreeft A, Tan IB, van den Brekel MWM, et al. The surgical dilemma of “functional inoperability” in oral and oropharyngeal cancer: current consensus on operability with regard to functional results. Clin Otolaryngol. 2009;34(2):140-146.
2. Gaige T A., Benner T, Wang R, et al. Three dimensional myoarchitecture of the human tongue determined in vivo by diffusion tensor imaging with tractography. J Magn Reson Imaging. 2007;26(3):654-661.
3. Tournier JD, Yeh CH, Calamante F, et al. Resolving crossing fibres using constrained spherical deconvolution: Validation using diffusion-weighted imaging phantom data. Neuroimage. 2008;42(2):617-625.
4. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870-888.
5. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(SUPPL. 1):208-219.
6. Froeling M, Nederveen AJ, Heijtel DFR, et al. Diffusion-tensor MRI reveals the complex muscle architecture of the human forearm. J Magn Reson Imaging. 2012;36(1):237-248.
7. Leemans A, Jeurissen B. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc Int Soc Magn Reson Med. 2009;245:3300.
8. Chang L-C, Jones DK, Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med. 2005;53:1088-1095.
9. Gilbert RJ, Wedeen VJ, Magnusson LH, et al. Three-dimensional myoarchitecture of the bovine tongue demonstrated by diffusion spectrum magnetic resonance imaging with tractography. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(11):1173-1182.
10. Taylor EN, Hoffman MP, Aninwene GE 2nd, et al. Patterns of Intersecting Fiber Arrays Revealed in Whole Muscle with Generalized Q-Space Imaging. Biophys J. 2015;108(11):2740-2749.
11. Takemoto H. Morphological Analyses of the Human Tongue Musculature for Three-Dimensional Modeling. J Speech, Lang Hear Res. 2001;44(1):95-107.
Figures