5005
Estimation of the Sensitivity Characteristics and Detection Capability of Diffusion-Weighted MR Sequences in Imaging Spontaneous Mechanical Activity in Musculature1Section on Experimental Radiology, University of Tuebingen, Tuebingen, Germany, 2Institute of Signal Processing and System Theory, University of Stuttgart, Stuttgart, Germany, 3Institute for Medical Psychology and Behavioural Neurobiology, University of Tuebingen, Tuebingen, Germany, 4Neurotechnology Laboratory, TECNALIA Health Department, San Sebastian, Spain, 5Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Spontaneous mechanical activity in musculature (SMAM) can be observed from time to time in diffusion-weighted images (DWI) of the human lower leg. In DWI, motion sensitivity is usually restricted to a time window between diffusion-sensitizing dephasing and rephrasing gradients. Capabilities to detect SMAM occurring outside this time window by DWI are expected to be clearly reduced. The temporal sensitivity of diffusion-weighted sequences to SMAM is evaluated by varying diffusion-sensitizing time. In addition, concurrent surface electromyography (sEMG) measurements were performed in order to reveal the temporal correlation of the events in both modalities.
Introduction
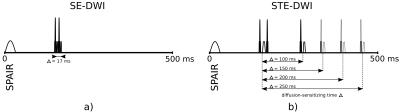
Series of diffusion-weighted (DW) images of the human lower leg can be impaired by spontaneous mechanical activities in musculature (SMAM) with random appearance in temporal and spatial domain1. It was shown that these SMAMs have a high correlation to electrical activity as measured by concurrent surface electromyography (sEMG)2,3 with a large variability in number of occurrences depending on the applied DW sequence4. A high accordance between detection capability and diffusion-sensitizing time is expected, but previous studies have shown a large difference between number of events in sEMG and SMAMs in DWI3. To provide more insight into the relation between diffusion-sensitizing time of DW sequences and the capability to image SMAMs, concurrent sEMG and DWI measurements with varying diffusion-sensitizing times (Fig.1) were carried out.Methods
Three volunteers (age: 36±14 years, BMI: 26.3±2.6 kg/m²) were examined with concurrent sEMG and DWI. MR acquisition: Series with 500 repetitions of transverse image were recorded at maximum diameter of the right calf with a prototype diffusion-weighted stimulated-echo EPI (STE-DWI) sequence with varying diffusion-sensitizing time and a diffusion-weighted Stejskal-Tanner spin-echo EPI (SE-DWI) sequence. Measurement parameters: matrix size: 64 x 64; FoV: 192 x 192 mm²; TE: 31 ms (SE-DWI: 41 ms); TR: 500 ms; BW: 2004 Hz/px; slice-thickness: 6 mm; diffusion-sensitizing time Δ: 100/150/200/250 ms (SE-DWI: Δ: 17 ms); b-value: 100 s/mm²; 6/8-readout; SPAIR for fat suppression. All images were acquired on a 3 T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with a 15-channel Tx/Rx-coil. sEMG acquisition: Concurrent sEMG measurements were recorded with an MR-compatible system (BrainAmp ExG MR, Brain Products GmbH, Gilching, Germany) at the same location: sampling rate: 5 kHz; inter-electrode distance: 2 cm; current-limiting resistor: 15 kΩ; bi-polar channels: 4; resolution: 0.5 µV. Electrodes were placed over the m. gastrocnemius medialis. Post-Processing: SMAMs in DWI were evaluated by an automated graph-based segmentation approach5. To correct for MR gradient switching induced artifacts in recorded sEMG measurement, artifact correction according to Niazy et al.6,7 was applied in EEGLAB8. To suppress physiological distortions, e.g. ballistocardiogram artifacts, sEMG signal was band-pass filtered (fbp = 20-500 Hz). For a robust detection of spontaneous events in sEMG, a semi-automated procedure based on a two-class support vector machine (600/600 training-dataset, radial-basis kernel, 10-fold cross-validation, grid-search optimized, 20 signal parameters for training9,10,11,12) was implemented based on Chang et al.13 with a subsequent human-observer decision to ensure detection reliability. Evaluation: Gross movements of the lower leg were discarded in both modalities. Number of events and event count maps (ECM) for different diffusion-sensitizing times were evaluated. For sensitivity estimation, SMAMs within a region of 3 cm around sEMG electrodes were assumed to be measureable. sEMG events with and without visible SMAM in DWI within a period of 500 ms were mapped separately with respect to the their occurrence in the TR interval.Results & Discussion
The automated sEMG detection achieved a test-accuracy 91.9 % in average.
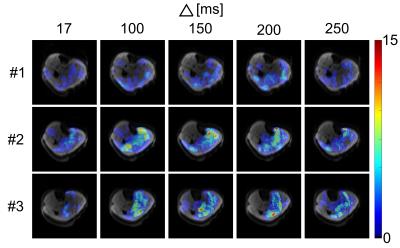
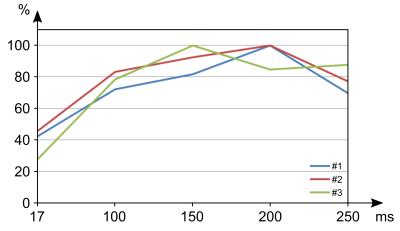
In Fig.2, ECMs of all volunteers show clear differences for DWI sequences with different diffusion-sensitizing times. Very short or very long diffusion-sensitizing time led to reduced sensitivity to SMAMs. Overall numbers of SMAMs were maximal for diffusion-sensitizing times Δ ranging from 150–200 ms (using a constant b-value = 100 s/mm2 for all measurements) (Fig.3). It was expected that very short Δ might lead to reduced sensitivity to SMAMs. The unexpected result that STE-DWI with very long Δ = 250 ms also led to reduced numbers of visible SMAMs compared to shorter Δ is possibly caused by complete relaxation of the muscle motions of a SMAM within the longer time intervals, and therefore complete rephasing of signals.
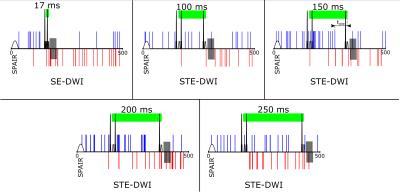
Temporal distributions of sEMG events with (blue) and without (red) visible SMAMs in the following DWI are indicated in Fig.4. It can be seen that especially sEMG events with longer delay time to the next motion sensitive period in the DWI sequence do not result in visible SMAMs. It must be considered that there is a temporal gap between the electrical activity (visible in sEMG) and force onset also for spontaneous unintended muscle activities leading to visible SMAMS in DWI. For active muscle contraction an electromechanical delay of 49.73±7.99 ms was reported in the literature14.
Conclusion
The temporal sensitivity characteristics were successfully estimated based on concurrent sEMG and DWI with varying diffusion-sensitizing time. A strong dependence between diffusion-sensitizing time and overall number of SMAMs visualized by DWI was clearly shown by this technique. Results are important for sequence optimization and for protocol standardization in MR imaging of SMAMs, and they give more insight in the temporal correlation of related electrical and mechanical activities.Acknowledgements
We thank Shiman, F., Institute for Medical Psychology and Behavioural Neurobiology, University of Tuebingen, and Erb, M., Biomedical Magnetic Resonance, University of Tuebingen, for their valuable technical support on this project.References
[1]: Steidle G, and Schick F.: "Addressing spontaneous signal voids in repetitive single-shot DWI of musculature: spatial and temporal patterns in the calves of healthy volunteers and consideration of unintended muscle activities as underlying mechanism". NMR Biomed 2015;28:810-10[2]: Schwartz, M. et al.: "Classification of signal voids in time-series of diffusion-weighted images of the lower leg by simultaneous MRI and EMG measurements: Initial findings". Proc. ISMRM 2016, Singapore
[3]: Schwartz, M. et al.: "Spontaneous activity in sEMG in relation to signal voids in repetitive single-shot DW-MRI of the human lower leg: Comparison of measurements under varying conditions". Proc. ESMRMB 2016, Vienna/Austria
[4] Schwartz, M et al.: "Temporal and spatial characteristics of signal voids in repetitive acquisitions of the human lower leg using spin-echo vs. stimulated echo diffusion-weighted imaging". Proc. ESMRMB 2016, Vienna/Austria
[5]: Schwartz, M. et al.: "Graph-based segmentation of signal voids in time series of diffusion-weighted images of musculature in the human lower leg". Proc. ISMRM 2016, Singapore
[6]: Niazy, R.K. et al.: "Removal of FMRI environment artifacts from EEG data using optimal basis sets". NeuroImage 2005;28(3):720-37
[7]: Ianetti, G.D. et al.: "Simultaneous recording of laser-evoked brain potentials and continuous, high-field functional magnetic resonance imagin in humans". NeuroImage 2005;32(3):1120-6
[8]: Delorme, A. and Makeig, S.: "EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis". J Neurosci Methods 2004;134(1):9-21
[9]: Lo Conte, L.R. et al.: "Hermite Expansions of Compact Support Waveforms: Applications to Myoelectric Signals". IEEE Trans on Biomed Eng 1994;41(12):1147-59
[10]: Merlo, A. et al.: "A Fast and Reliable Technique for Muscle Activity Detection From Surface EMG Signals". IEEE Trans Biomed Eng 2003;50(3):316-23
[11]: Kaiser, J.F.: "On a simple algorithm to calculate the 'energy' of a signal". IEEE ICASSP 1993:149-152
[12]: Huang, N.E. et al.: "The empirical mode decomposition and the Hilbert spectrum for non-linear and non stationary time series analysis". Proc. Royal Soc. London A 1998;454:903-95
[13]: Chang, C.-C and Lin, C.-J.: "LIBSVM: a library for support vector machines". ACM TIST 2011;2:27:1—27:27
[14]: Begovic, H et al.: "Detection of the electromechanical delay and its components during voluntary isometric contraction of the quadriceps femoris muscle". Front Physiol 2014;5:494
Figures