4989
Chemical Shift Effect Predicting Lymph Node Status in Rectal Cancer using High-Resolution MR Imaging with Node-for-node Matched Histopathological Validation1Department of Diagnostic Radiology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People's Republic of China
Synopsis
To evaluate the value of chemical shift effect (CSE), as well as other criteria for the prediction of lymph node status. Lymph nodes harvested from transversely whole-mount specimens were compared with in vivo and ex vivo images to obtain MR characteristics including CSE, as well as other predictors of 255 benign and 35 metastatic nodes. Our results revealed that CSE is a reliable predictor for differentiating benign from metastatic lymph nodes. Other predictors of nodal location, border, signal intensity and minimum distance to rectal wall were also proved to be useful for the diagnosis.
Objectives
To evaluate the value of chemical shift effect (CSE), as well as other criteria for the prediction of lymph node status.Materials and Methods
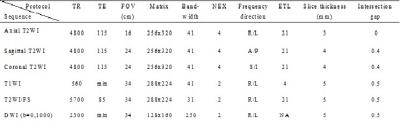
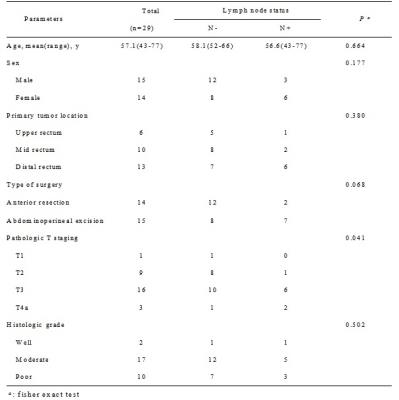
Twenty-nine patients who underwent radical surgery of rectal cancers were studied with preoperative and postoperative specimen MRI. Lymph nodes were harvested from transversely whole-mount specimens, and compared with in vivo and ex vivo images to obtain a precise slice-for-section match. Preoperative MR characteristics including CSE, as well as other predictors were evaluated by two readers independently between benign and metastatic nodes.Results
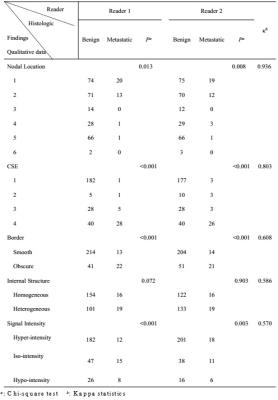
255 benign and 35 metastatic nodes were obtained for node-by-node analysis. 71.4% and 69.4% of benign nodes were detected with regular CSE for two readers respectively, whereas 80% and 74.3% of metastatic nodes with absence of CSE. The inter-rater agreement was excellent between readers (κ=0.803). The predictors of nodal location, border, signal intensity and minimum distance to rectal wall were also proved to be useful for the diagnosis but with AUCs lower than that of CSE.Conclusions
CSE is a reliable predictor for differentiating benign from metastatic lymph nodes. Additional criteria are needed to be taken into account when it is difficult to determine the nodal status by using only a single predictor.Acknowledgements
No acknowledgement found.References
1. Nougaret S, Reinhold C, Mikhael HW, Rouanet P, Bibeau F, Brown G (2013) The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the "DISTANCE"? Radiology 268: 330-344.
2. Lambregts DM, Lahaye MJ, Heijnen LA, et al (2016) MRI and diffusion-weighted MRI to diagnose a local tumour regrowth during long-term follow-up of rectal cancer patients treated with organ preservation after chemoradiotherapy. Eur Radiol 26: 2118-2125.
3. Althumairi AA, Gearhart SL (2015) Local excision for early rectal cancer: transanal endoscopic microsurgery and beyond. J Gastrointest Oncol 6:296-306.
4. Kim YC, Kim JK, Kim MJ, Lee JH, Kim YB, Shin SJ (2016) Feasibility of mesorectal vascular invasion in predicting early distant metastasis in patients with stage T3 rectal cancer based on rectal MRI. Eur Radiol 26:297-305.
5. Brown G, Richards CJ, Bourne MW, et al (2003) Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 227:371-377.
6. Lahaye MJ, Beets GL, Engelen SM, et al (2009) Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes? Radiology 252:81-91.
7. Furey E, Jhaveri KS (2014) Magnetic resonance imaging in rectal cancer. Magn Reson Imaging Clin N Am 22:165-190.
8. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J (2004) Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology 232:773-783.
9. Alberda WJ, Dassen HP, Dwarkasing RS, et al (2013) Prediction of tumor stage and lymph node involvement with dynamic contrast-enhanced MRI after chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis 28:573-580.
10. Lahaye MJ, Engelen SM, Nelemans PJ, et al (2005) Imaging for predicting the risk factors—the circumferential resection margin and nodal disease—of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR 26: 259–268.
11. Chun HK, Choi D, Kim MJ, et al (2006) Preoperative staging of rectal cancer: comparison of 3-T high-field MRI and endorectal sonography. AJR. American J Roentgenology 187:1557-1562.
12. Akasu T, Linuma G, Takawa M, Yamamoto S, Muramatsu Y, Moriyama N (2009) Accuracy of high-resolution magnetic resonance imaging in preoperative staging of rectal cancer. Ann Surg Oncol 16:2787-2794.
13. Lambregts DM, Maas M, Riedl RG, et al (2011) Value of ADC measurements for nodal staging after chemoradiation in locally advanced rectal cancer-a per lesion validation study. Eur Radiol 21: 265-273. 14. Heijnen LA, Lambregts DM, Mondal D, et al (2013) Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol 23:3354-3360.
15. Attenberger UI, Pilz LR, Morelli JN, et al (2014) Multi-parametric MRI of rectal cancer - do quantitative functional MR measurements correlate with radiologic and pathologic tumor stages? Eur J Radiol 83:1036-1043.
16. Lahaye MJ, Engelen SM, Kessels AG, et al (2008) USPIO-enhanced MR imaging for nodal staging in patients with primary rectal cancer: predictive criteria. Radiology 246:804-811.
17. Kneissl S, Probst A (2006) Magnetic resonance imaging features of presumed normal head and neck lymph nodes in dogs. Vet Radiol Ultrasound 47:538-541.
18. Lambregts DM, Heijnei LA, Maas M, et al (2013) Gadofosveset-enhanced MRI for the assessment of rectal cancer lymph nodes: predictive criteria. Abdom Imaging 38:720-727.
19. Farshchian N, Tamari S, Farshchian N, Madani H, Rezaie M, Mohammadi-Motlagh HR (2011) Diagnostic value of chemical shift artifact in distinguishing benign lymphadenopathy. Eur J Radiol 80:594-597.
20. Stadler A, Schima W, Ba-Ssalamah A, Kettenbach J, Eisenhuber E (2007) Artifacts in body MR imaging: their appearance and how to eliminate them. Eur Radiol 17:1242-1255.
21. Ohtani O, Ohtani Y (2008) Structure and function of rat lymph nodes. Arch Histol Cytol 71:69-76. 22. Kamperdijk EW, Dijkstra CD, Döpp EA (1987) Transport of immune complexes from the subcapsular sinus into the lymph node follicles of the rat. Immunobiology 174:395-405.
23. Diaz LK, Hunt K, Ames F, et al (2003) Histologic localization of sentinel lymph node metastases in breast cancer. Am J Surg Pathol 27:385-389.
24. Hayashi K, Jiang P, Yamauchi K, et al (2007) Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cance Res 67:8223-8228.
25. Servais EL, Colovos C, Bograd AJ, White J, Sadelain M, Adusumilli PS (2011) Animal models and molecular imaging tools to investigate lymph node metastases. J Mol Med (Berl) 89:753-769.
26. Zhou J, Zhan S, Zhu Q, et al (2014) Prediction of nodal involvement in primary rectal carcinoma without invasion to pelvic structures: accuracy of preoperative CT, MR, and DWIBS assessments relative to histopathologic findings. PloS one 9:e92779.
27. Engelen SM, Beets-Tan RG, Lahaye MJ, Kessels AG, Beets GL (2008) Location of involved mesorectal and extramesorectal lymph nodes in patients with primary rectal cancer: preoperative assessment with MR imaging. Eur J Surg Oncol 34:776-781.
28. Glasgow SC, Bleier JI, Burgart LJ, Finne CO, Lowry AC (2012) Meta-analysis of histopathological features of primary colorectal cancers that predict lymph node metastases. J Gastrointest Surg 16:1019-1028.
29. Park JS, Jang YJ, Choi GS, et al (2014) Accuracy of preoperative MRI in predicting pathology stage in rectal cancers: node-for-node matched histopathology validation of MRI features. Dis Colon Rectum 57: 32-38.
30. Yeo KH, Kim HH, Kim DY, Kim YJ, Ju JK (2014) A distribution weighted prognostic scoring model for node status in advanced rectal cancer. Cancer Res Treat 46(1): 41-47.
Figures