4983
Optimization of high b values for intravoxel incoherent motion imaging of rectal cancer : a pilot study1Department of Radiology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People's Republic of China
Synopsis
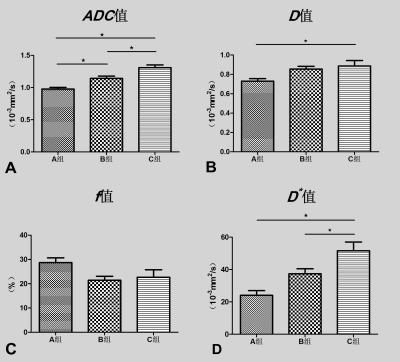
To optimize the high b values (>200s/mm2) for intravoxel incoherent motion imaging of rectal cancer and to observe the effect of high b values variation on IVIM parameters. Three groups (A group with all 16 b values: 0,10,20,30,40,60,80,100,150,200,400,800,1000,1200,1500,2000, B group with 14 b values: 0,10,20,30,40,60,80,100,150,200,400,800,1000,1200 and C group with 12 b values: 0,10,20,30,40,60,80,100,150,200,400,800) were selected respectively for measurement by a radiologist. The average values of each measurement were used for statistical analysis. One-way analysis of variance (ANOVA) and post-hoc test were performed on the mean values of IVIM parameters in groups A, B, and C, with a significance level of P<0.05. The p values of ANVOA results in ADC、D、D* values were less than 0.05, the differences were statistically significant. The p values of Bonferroni post-hoc test in D、D*、f values were not statistically significant differences in group A and B. With the number of high b values decrease, the values of ADC、D、D* values and standard error were increased, while of f values was not changed significantly. In our study, the reproducibility of the IVIM parameters caused by high b value variation was not significant. The value of selected b > 1500 need to be further studied.
Objective
To optimize the high b values (>200s/mm2) for intravoxel incoherent motion imaging of rectal cancer and to observe the effect of high b values variation on IVIM parameters.Methods
Twenty two patients with rectal adenocarcinoma were included the prospective study. Three groups (A group with all 16 b values: 0,10,20,30,40,60,80,100,150,200,400,800,1000,1200,1500,2000, B group with 14 b values: 0,10,20,30,40,60,80,100,150,200,400,800,1000,1200 and C group with 12 b values: 0,10,20,30,40,60,80,100,150,200,400,800) were selected respectively for measurement by a radiologist (with 10 years of experience in gastrointestinal cancer imaging). The largest slice of the solid components of the tumor was selected for measurement in the IVIM metrics map (b=800). Monoexponential ADC and biexponential IVIM metrics maps and IVIM parameters were generated automatically by the software. The same measurement were repeated after one week apart. The average values of each measurement were used for statistical analysis. The consistency and reproducibility of the two measurements were analyzed by Bland-Altman plots. One-way analysis of variance (ANOVA) and post-hoc test were performed on the mean values of IVIM parameters in groups A, B, and C, with a significance level of P<0.05.Results
The D* values variations of 95% limits of agreement (LoA) between groups A, B and A, C were relatively large. The 95% LoA of ADC、D values between groups were not obvious. The p values of ANVOA results in ADC、D、D* values were less than 0.05, the differences were statistically significant. The p value of ANVOA results in f values was greater than 0.05, the difference was not statistically significant. The p values of Bonferroni post-hoc test in D、D*、f values were not statistically significant differences in group A and B. With the number of high b values decrease, the values of ADC、D、D* values and standard error were increased, while of f values was not changed significantly.Conclusions
In our study, the reproducibility of the IVIM parameters caused by high b value variation was not significant. The value of selected b > 1500 need to be further studied.Acknowledgements
No acknowledgement found.References
[1]. Siegel, R.L., K.D. Miller and A. Jemal, Cancer statistics, 2016. CA Cancer J Clin, 2016. 66(1): p. 7-30.
[2]. Chen, W., et al., Cancer statistics in China, 2015. CA Cancer J Clin, 2016. 66(2): p. 115-32.
[3]. Xie, H., et al., Effectiveness of the apparent diffusion coefficient for predicting the response to chemoradiation therapy in locally advanced rectal cancer: a systematic review and meta-analysis. Medicine (Baltimore), 2015. 94(6): p. e517.
[4]. Birlik, B., et al., Diffusion-weighted MRI and MR- volumetry--in the evaluation of tumor response after preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Magn Reson Imaging, 2015. 33(2): p. 201-12.
[5]. Monguzzi, L., et al., Locally advanced rectal cancer: value of ADC mapping in prediction of tumor response to radiochemotherapy. Eur J Radiol, 2013. 82(2): p. 234-40.
[6]. Choi, M.H., et al., Diffusion-weighted imaging: Apparent diffusion coefficient histogram analysis for detecting pathologic complete response to chemoradiotherapy in locally advanced rectal cancer. J Magn Reson Imaging, 2016. 44(1): p. 212-20.
[7]. Le Bihan, D., et al., Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology, 1988. 168(2): p. 497-505.
[8]. Iima, M. and D. Le Bihan, Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology, 2016. 278(1): p. 13-32.
[9]. Yu, X.P., et al., Discrimination between Metastatic and Nonmetastatic Mesorectal Lymph Nodes in Rectal Cancer Using Intravoxel Incoherent Motion Diffusion-weighted Magnetic Resonance Imaging. Acad Radiol, 2016. 23(4): p. 479-85.
[10]. Nougaret, S., et al., Intravoxel Incoherent Motion-derived Histogram Metrics for Assessment of Response after Combined Chemotherapy and Radiation Therapy in Rectal Cancer: Initial Experience and Comparison between Single-Section and Volumetric Analyses. Radiology, 2016. 280(2): p. 446-54.
[11]. Qiu, L., et al., Role of quantitative intravoxel incoherent motion parameters in the preoperative diagnosis of nodal metastasis in patients with rectal carcinoma. J Magn Reson Imaging, 2016.
[12]. Bauerle, T., et al., Diffusion-weighted imaging in rectal carcinoma patients without and after chemoradiotherapy: a comparative study with histology. Eur J Radiol, 2013. 82(3): p. 444-52.
[13]. Freiman, M., et al., In vivo assessment of optimal b-value range for perfusion-insensitive apparent diffusion coefficient imaging. Med Phys, 2012. 39(8): p. 4832-9.
[14]. Karki, K., et al., Estimation of optimal b-value sets for obtaining apparent diffusion coefficient free from perfusion in non-small cell lung cancer. Phys Med Biol, 2015. 60(20): p. 7877-91.
[15]. Leporq, B., et al., Optimization of intra-voxel incoherent motion imaging at 3.0 Tesla for fast liver examination. J Magn Reson Imaging, 2015. 41(5): p. 1209-17.
[16]. Zhang, J.L., et al., Optimization of b-value sampling for diffusion-weighted imaging of the kidney. Magn Reson Med, 2012. 67(1): p. 89-97.
[17]. Cohen, A.D., et al., The effect of low b-values on the intravoxel incoherent motion derived pseudodiffusion parameter in liver. Magn Reson Med, 2015. 73(1): p. 306-11. [18]. Kim, J.H., et al., Diffusion-Related MRI Parameters for Assessing Early Treatment Response of Liver Metastases to Cytotoxic Therapy in Colorectal Cancer. AJR Am J Roentgenol, 2016. 207(3): p. W26-32.
[19]. Fujima, N., et al., Prediction of the treatment outcome using intravoxel incoherent motion and diffusional kurtosis imaging in nasal or sinonasal squamous cell carcinoma patients. Eur Radiol, 2016. [20]. Kakite, S., et al., Hepatocellular carcinoma: short-term reproducibility of apparent diffusion coefficient and intravoxel incoherent motion parameters at 3.0T. J Magn Reson Imaging, 2015. 41(1): p. 149-56.
[21]. Lee, Y., et al., Intravoxel incoherent motion diffusion-weighted MR imaging of the liver: effect of triggering methods on regional variability and measurement repeatability of quantitative parameters. Radiology, 2015. 274(2): p. 405-15.
[22]. Rydhog, A.S., et al., Intravoxel incoherent motion (IVIM) imaging at different magnetic field strengths: what is feasible? Magn Reson Imaging, 2014. 32(10): p. 1247-58.
[23]. Shirota, N., et al., Intravoxel incoherent motion MRI as a biomarker of sorafenib treatment for advanced hepatocellular carcinoma: a pilot study. Cancer Imaging, 2016. 16: p. 1.
[24]. Hou, J., et al., Value of intravoxel incoherent motion and dynamic contrast-enhanced MRI for predicting the early and short-term responses to chemoradiotherapy in nasopharyngeal carcinoma. Medicine (Baltimore), 2016. 95(35): p. e4320.