4978
Dynamic Contrast-Enhanced Imaging of the Rectum Using Golden-Angle Radial Sparse Parallel MRI (GRASP): Initial Experience and Comparison to a Conventional Approach Using Time-resolved Angiography With Interleaved Stochastic Trajectories (TWIST).1Institute of Clinical Radiology and Nuclear Medicine, University Medicine Mannheim, Mannheim, Germany, 2Department of Radiology, Peking Universtiy First Hospital, Beijing, China, 3Siemens Healthcare, Erlangen, Germany, 4Department of Radiology, New York University, New York City, NY, USA
Synopsis
MR perfusion images to discriminate between normal rectal wall and rectal cancers with less variance of perfusion values and superior image quality compared to conventional TWIST-Angiography can be generated using time-resolved free-breathing MRI with continuous golden-angle radial sampling and iterative reconstruction (GRASP). Additional morphologic assessment (“one-stop-shop”) with high spatial resolution, artifact-insensitive, multiphase, contrast-enhanced imaging may increase accuracy and diagnostic confidence of the examination.
Introduction
Multiparametric MRI has evolved to a standard method for both rectal cancer staging and evaluation of response to therapy after neoadjuvant chemoradiation (CRT) (1-4). MR perfusion (MRPF) may be useful as a MRI-based biomarker for the prediction of therapeutic response (5). Unfortunately, standard highly–accelerated sequences, such as the keyhole technique Time-resolved angiography With Interleaved Stochastic Trajectories (TWIST), are prone to artifacts such as bowel motion, which impair the diagnostic accuracy. Moreover, the view-sharing approach results in a relatively large temporal footprint. Recently, golden-angle radial sparse parallel MRI (GRASP) has been proposed (6), which combines a golden-angle radial acquisition with a compressed-sensing reconstruction technique to improve temporal resolution. GRASP has already been shown useful for motion-insensitive liver and renal imaging in previous studies (6, 7). Besides the option to create parametric perfusion maps, GRASP in contrast to TWIST allows for the acquisition of detailed morphologic images. To our knowledge, this is the first study to systematically compare GRASP and TWIST in a rectal cancer and healthy rectum cohort.Materials and Methods
Twenty rectal cancer patients underwent a pelvic MR examination in our hospital. In 10 patients MRPF was conducted using GRASP, and in 10 patients using TWIST. In both cohorts, 5 patients were examined prior and 5 post CRT. 20 patients (10 GRASP; 10 TWIST) without rectal pathologies served as healthy control group. All MR images were acquired using a 3 T MR scanner (MAGNETOM Trio/Skyra, Siemens Healthcare, Erlangen, Germany). TWIST images were obtained at a temporal resolution of 4.8s. For GRASP, 1586 radial spokes were continuously acquired within 185 seconds using a prototype golden-angle radial stack-of-stars 3D gradient-echo sequence at 3.45s temporal resolution. Two radiologists (Reader 1 with 7 years and Reader 2 with 3 years of experience in abdominal MRI) independently rated the quality of morphologic images and perfusion maps, artifacts, quality of arterial input functions (AIFs) according to a Likert-type scale. The AIF was determined by placing a region of interest (ROI) in the right femoral artery. After calculation of PF maps using UMMperfusion (8), 2 ROIs were placed in bilateral obturator internus muscle, 3 (upper, middle and lower part) in normal recta and another 3 in rectal cancers in each dataset to obtain PF values. Results were averaged for statistical evaluation. Independent-t-tests for both assessment of image quality and artifacts, as well as to compare GRASP- and TWIST-derived PF values in both patients with rectal cancer and normal rectum were applied. Additionally, differences between PF values obtained with GRASP and TWIST in rectal cancers and remaining normal rectal wall were assessed and compared using paired t-tests. Figure 1 details the workflow.Results
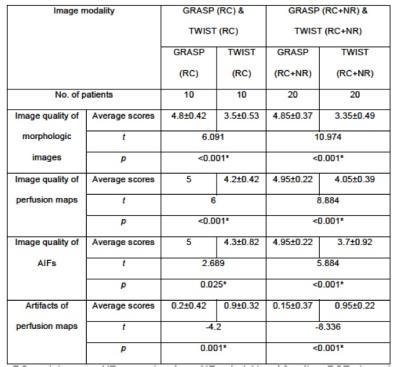
Significantly higher scores were obtained by GRASP for image quality, quality of perfusion maps, and AIFs. Artifacts of perfusion maps were rated significantly lower compared to TWIST (Figures 2, 3). The PF of obturator internus muscle in the healthy GRASP cohort was 13.87±7.69 ml/100ml/min, compared to 112.42±127.35 ml/100ml/min in the healthy TWIST cohort.The PF of normal rectal wall derived by GRASP was 31.78±7.39 ml/100ml/min, compared to 127.22±121.8 ml/100ml/min in TWIST perfusions (Figure 4). One post-CRT patient of the TWIST and GRASP cohort showed diffuse rectal tumor involvement and was excluded from the evaluation of normal wall. The PF of primary rectal cancers using GRASP was 60.67±23.83 ml/100ml/min, compared to 141.03±59.44 ml/100ml/min after CRT. Significant differences were obtained between rectal cancer and residual normal wall in patients prior to and after CRT (prior to CRT: t = -3.388, p = 0.004< 0.05; after CRT: t = -5.595, p < 0.001). The PF of primary rectal cancers obtained by TWIST was 44.85±17.86 ml/100ml/min, compared to 84.73±28.15 ml/100ml/min after CRT. Significant differences were obtained between rectal cancer and residual normal wall both prior to and post CRT (Prior to CRT: t = 4.493, p = 0.001; After CRT: t = 2.657, p = 0.022 < 0.05) (Figure 5).Discussion
Our study has several limitations. First, GRASP currently requires a long reconstruction time of about 2 hours, which could be reduced, e.g., by utilizing GPUs. The small number of subjects limits the applicability of our findings. The absolute differences in PF between GRASP and TWIST may be attributed to different acquisition parameters, but should be evaluated in more detail. Nevertheless, our results indicate that reproducible perfusion values can be generated using GRASP with less variance (indicated by results in normal rectal wall and muscle) and higher image quality compared to TWIST, which enables discrimination between normal rectal wall and rectal cancers. High-resolution, motion-insensitive morphological assessment adds to the confidence of this "one-stop-shop"-examination.Acknowledgements
No acknowledgement found.References
1. Nougaret S, Rouanet P. Restaging rectal cancer after neoadjuvant treatment with multiparametric MRI: A landscape of new opportunities. Diagn Interv Imaging. 2016;97(9):839- 41.
2. Hotker AM, Tarlinton L, Mazaheri Y, Woo KM, Gonen M, Saltz LB, et al. Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: A comparison of morphological, volumetric and functional MRI parameters. European radiology. 2016.
3. Nie K, Shi L, Chen Q, Hu X, Jabbour SK, Yue N, et al. Rectal Cancer: Assessment of Neoadjuvant Chemoradiation Outcome based on Radiomics of Multiparametric MRI. Clin Cancer Res. 2016.
4. Cerny M, Dunet V, Prior JO, Hahnloser D, Wagner AD, Meuli RA, et al. Initial Staging of Locally Advanced Rectal Cancer and Regional Lymph Nodes: Comparison of Diffusion-Weighted MRI With 18F-FDG-PET/CT. Clinical nuclear medicine. 2016;41(4):289-95.
5. Oberholzer K, Menig M, Pohlmann A, Junginger T, Heintz A, Kreft A, et al. Rectal cancer: Assessment of response to neoadjuvant chemoradiation by dynamic contrast- enhanced MRI. J Magn Reson Imaging. 2013;38(1):119-26.
6. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu L, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible volumetric MRI. Magn Reson Med. 2014; 72(3):707-17.
7. Riffel P, Zoellner FG, Budjan J, Grimm R, Block TK, Schoenberg SO, et al. "One-Stop Shop": Free-Breathing Dynamic Contrast-Enhanced Magnetic Resonance Imaging of the Kidney Using Iterative Reconstruction and Continuous Golden-Angle Radial Sampling. Investigative radiology. 2016;51(11):714-9.
8. Zöllner FG, Weisser G, Reich M, Kaiser S, Schoenberg SO, Sourbron SP, Schad LR. UMMPerfusion: an open source software tool towards quantitative MRI perfusion analysis in clinical routine.J Digit Imaging. 2013 Apr;26(2):344-52.
Figures
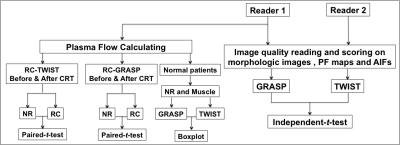
Workflow-chart of data analysis. PF: plasma flow. RC: rectal cancer. NR: normal rectum. AIF: arterial input function. CRT: chemoradiation.

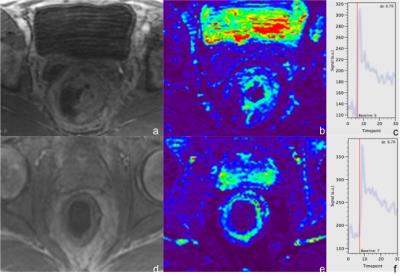
a-c: TWIST images of a primary rectal cancer. a: contrast-enhanced TWIST image, the image quality was rated moderate (3); b: perfusion map of the same anatomical location, the image quality was rated good (4) with slight artifacts. c: AIF of TWIST, the quality was rated 4. d-f: TWIST images of a rectal cancer after CRT. d: contrast- enhanced TWIST image, the image quality was rated 4; e: perfusion map of the same anatomical location, the image quality was rated 4 with slight artifacts. f: AIF of TWIST, the quality was rated 3.
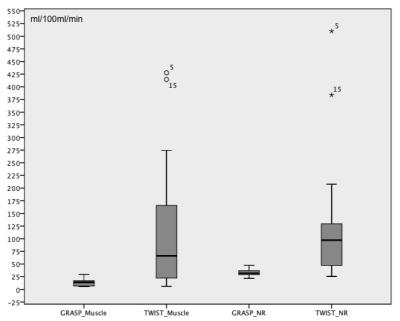
Boxplot of PF of obturator internus muscle and normal rectal wall obtained by GRASP and TWIST. Y-axis stands for the plasma flow. NR: normal rectum.