4977
Pretreatment diffusion kurtosis imaging for predicting the response of locally advanced rectal cancer to neoadjuvant chemoradiation therapy1Radiology, China-Japan Friendship Hospital, Beijing, People's Republic of China, 2Philips Healthcare China, Beijing, People's Republic of China
Synopsis
Diffusion kurtosis imaging (DKI) is an emerging technique, which could reflect restricted water diffusion within the complex microstructure of most tissues based on non-Gaussian diffusion model. It has been reported that DKI was used in central system diseases, tumor grade, even assessment of treatment response. However, there is limited research reported about the clinical application of DKI in rectal cancer, and the value of DKI in monitoring rectal cancer treatment was still unclear.
Synopsis
Neoadjuvant chemoradiation therapy (CRT) followed by surgery has been established as the standard for locally advanced rectal cancer1. The treatment response after CRT is normally evaluated by MRI. However, MRI morphology techniques suffer from limitations in the interpretation of fibrotic scar tissue and inflammation. Diffusion kurtosis imaging (DKI) is an emerging technique, which could reflect restricted water diffusion within the complex microstructure of most tissues based on non-Gaussian diffusion model2. It has been reported that DKI was used in central system diseases3-5, tumor grade6, even assessment of treatment response7-9. However, there is limited research reported about the clinical application of DKI in rectal cancer, and the value of DKI in monitoring rectal cancer treatment was still unclear.Purpose
To retrospectively evaluate the role of pretreatment diffusion kurtosis imaging (DKI) in differentiating patients with locally advanced rectal cancers who will respond to long-course chemoradiation therapy (CRT) from those who will not respond, with tumor regression grade (TRG) obtained by postoperative pathological results as the reference standard.Methods
Forty-seven patients who underwent pretreatment pelvis magnetic resonance imaging (MRI) examination followed by CRT and surgery were enrolled in the study. All pelvis MRI examinations were performed in 3.0T MR unit with diffusion-weighted imaging (DWI) sequences. DWI sets with the corresponding HR-T2WI available for anatomic reference. Totally, seven b values (0, 400, 800 1000, 1200, 1500 and 2000s/mm2) were adopted and DKI derived parameters (MD, mean diffusivity; MK, mean kurtosist; FA, fractional anisotropy) were measured independently by two radiologists using software. The DKI parameters between CRT responders and non-responders were compared by using independent samples t test or Kolmogorov-Smirnov test, and relevant diagnostic performance in the prediction of the response to CRT was evaluated by receiver operating characteristic (ROC) analysis. The area under the ROC curve (AUC) and the optimal cut-off values were calculated, meanwhile accuracy rate, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) was determined. Correlations between TRG and DKI derived parameters were also respectively analyzed using Spearman’s correlation coefficients. Interobserver agreement of DKI derived parameter were evaluated using the intraclass correlation coefficient (ICC). P<0.05 was considered to indicate a statistically significant difference.Results
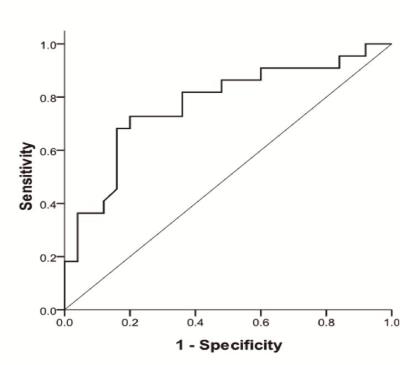
The two readers showed relatively good interobserver agreement (ICC=0.7109-0.9004; narrow with of 95% limits of agreement). 22 patients showed response to CRT and no-response was noted in 25 patients. MD values were significantly lower in CRT responders than in non-responders (p=0.046), while MK and FA values showed different trend (CRT responders: MK =0.97±0.19, FA=0.16±0.08; non-responders: MK =0.87±0.16, FA=0.09±0.05) (Table 1). According to ROC curve, FA values demonstrated the higher AUC (0.774), while MD presented with the lower AUC value (0.655). The optimal cutoff values for FA and MD were 0.127 (FA values of CRT responders was greater than this value; accuracy rate 68.09%, sensitivity 63.64%, specificity 72.00%, PPV 66.67%, NPV 69.23% ), 1.095×10-3mm2/s (MD values of CRT responders was lower than this value; accuracy rate 59.57%, sensitivity 59.09%, specificity 60.00%, PPV 56.52%, NPV 62.50% ), respectively. In addition, a moderate negative correlation between pretreatment FA and postoperative TRG was found (r=-0.370, p=0.01 ).Conclusion
Pretreatment DKI parameters, especially FA, might be valuable non-invasive index to evaluate response to CRT in locally advanced rectal cancer.Acknowledgements
No acknowledgement found.References
1. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731e40.
2. Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432-1440.
3. Raab P, Hattingen E, Franz K, et al. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology 2010;254:876–881.
4. Kamagata K, Tomiyama H, Motoi Y, et al. Diffusional kurtosis imaging of cingulate fibers in Parkinson disease: comparison with conventional diffusion tensor imaging. Magn Reson Imaging 2013;31:1501–1506.
5. Lee CY, Tabesh A, Spampinato MV, et al. Diffusional kurtosis imaging reveals a distinctive pattern of microstructural alternations in idiopathic generalized epilepsy. Acta Neurol Scand 2014;130:148–155.
6. Yan B, Yusong L, Jie T, et al. Grading of gliomas by using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR imaging. Radiology. 2016; 278(2): 496-504.
7. Rosenkrantz AB, Sigmund EE, Winnick A, et al. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liverexplants. Magn Reson Imaging 2012;30:1534-1540.
8. Goshima S, Kanematsu M, Noda Y, et al. Diffusion kurtosis imaging to assess response to treatment in hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 2015; 204:W543-549.
9. Chen Y, Ren W, Zheng D, et al. Diffusion kurtosis imaging predicts neoadjuvant chemotherapy responses within 4 days in advanced nasopharyngeal carcinoma patients. J Magn Reson Imaging JMRI. 2015;42(5):1354-61.