4966
Metabolic Imaging of β3-adrenoreceptor Activated BAT and its Systemic Effect on Abdominal Fat in Diet Induced Obese Model1Laboratory of Metabolic Imaging, Singapore Bioimaging Consortium, A*STAR, Singapore, 2Singapore Institute for Clinical Sciences, A*STAR, Singapore
Synopsis
Imbalance in dietary intake and energy expenditure are associated with obesity, diabetes and metabolic disorders. Adipocyte size and expansion of adipose tissue plays a critical role towards the progression of diet induced obesity. Brown adipose tissue (BAT) plays a critical role in modulating different fat depots in the body. BAT can be functionally activated by administering the β3-adrenergic agonist. Understanding the mechanisms associated with BAT activation and the possibility of reversing insulin resistance and its impact on whole body metabolism is of current clinical interest for combating diabetes. In the current study, we investigated the fat partitioning in high fat diet induced obese rodent model by β-adrenergic-mediated BAT activation.
Purpose
Investigation of abdominal fat partitioning in high fat diet induced obese model by β3-adrenergic-mediated BAT activation and its systemic effect on reversal of whole body insulin sensitivityIntroduction
Obesity and diabetes are major metabolic disorders associated with dietary intake and sedentary life style [1-2]. Brown adipose tissue (BAT) is capable of increasing thermogenesis and can play a critical role in modulating different fat depots in the body. The β3-adrenergic receptors are abundantly expressed in BAT and it can be activated by administering the β3-adrenergic agonist [3-4]. In addition, β3-agonist can also remodel the white adipose tissue (WAT) into Beige fat. There is a large interest in understanding the mechanisms associated with BAT activation and the possibility of reversing insulin resistance, and its impact on whole body metabolism. In the current study, we investigated the fat partitioning in high fat diet induced obese rodent model by β3-adrenergic-mediated BAT activation.Methods
All the experimental procedures carried out in the current study were in compliance and approved by institutional animal care and use committee. Eight weeks old, male Wister rats were randomized into two groups (Gp1 and Gp2). The Gp1 (n=12) and Gp2 (n=12) animals were fed with high fat diet (HFD) and chow diet (CD) for four weeks respectively. After the diet intervention each group were further divided into two sub groups and treated with either β3-adrenergic agonist, CL-316243 at 0.1mg/Kg body weight or equal volume of saline for 14 days. Longitudinal MR imaging was performed on 7T scanner (Bruker) using 72 mm volume transmit and 2x2 phased array receive only coils. Two point Dixon imaging was performed in interscapular brown adipose tissue (iBAT) and also in the abdomen. Imaging parameters used for iBAT imaging: FOV 54x54 mm2, matrix size 256×256, resolution 211μmx211μm, ST 1mm, TR 8ms, average 1, flip angle 8°, bandwidths of 1090 and 1500Hz/pixel, TE1/TE2 1/2.5 ms. Imaging parameters for abdomen are similar as used for iBAT except, FOV of 68x68 mm2,resolution 266μmx266μm. After terminal experiments, BAT and abdominal fat tissue samples were fixed in 10% neutral buffered solution for 24 hours. Hematoxylin (H) and eosin (E) staining was performed on 5µm tissue section.Results and Discussion
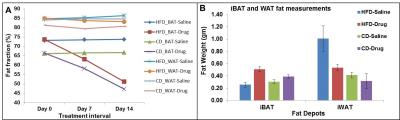
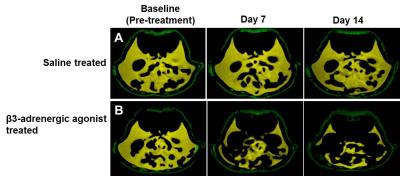
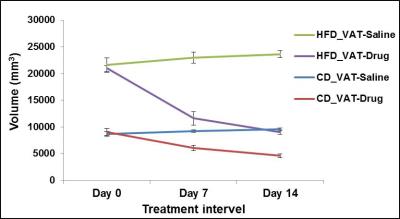
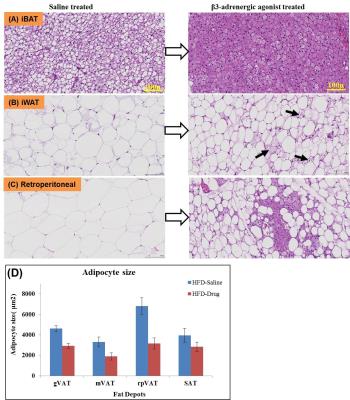
Figures 1A, B show the iBAT fat fraction maps from high fat (A) and chow (B) diet fed animal prior to BAT activation (day 0) and post BAT activation (day 7 and day 14) by β3-adrenergic agonist administration. Quantitative changes in fat content in iBAT and WAT from chow and high fat diet fed animals at prior and post BAT activation are shown in Figure 2A. The FF of iBAT in CD fed group at day 0 was 66.39±1.51%, which reduced to 58± 2.5% and 47.20±2.6% at day 7 and 14 respectively post activation. The FF of iBAT in HFD fed group on day 0 was 73.67±2.3%, which reduced to 63.1±1.5% and 51.1±1.2% respectively at day 7 and 14 post activation. There were no significant changes in FF of animals in saline treated groups. Reduction of fat content after activation implies the lipolysis of lipids into free fatty acids utilized in beta oxidation within mitochondria. The β3-adrenergic agonist (CL 316243) is known to activate the BAT through the stimulation of sympathetic nervous system. The CPTI mRNA levels were up-regulated in activated iBAT in β3-adrenergic agonist treated CD and HFD fed animals due to enhanced beta oxidation. The UCP1 mRNA levels were also up-regulated in iBAT of these animals due to enhanced thermogenesis. The weight of iBAT increased whereas the weight of iWAT decreased with β3-adrenergic agonist treatment in both chow and high fat diet groups (Figure 2B) indicating potential remodeling of WAT into BAT within the interscapular area. The BAT also functions as an endocrine organ by secreting the endocrine factors to modulate the whole body fat metabolism [5]. We studied the abdominal fat metabolism to understand the systemic effects of iBAT on body fat. Figure 3A,B shows the segmented visceral (yellow) and subcutaneous (green) abdominal fat volumes prior (day 0) and post BAT activation (day 7 & day 14) from high fat diet fed obese animals. Visceral and subcutaneous fat volumes in chow and high fat diet (Figure 4) fed animals were significantly reduced in β3-adrenergic agonist treated group compared to saline treated group. Reduction in abdominal fat depots including gonadal, mesenteric, retroperitoneal were further confirmed by performing fat pad measurements. The lipolysis induced beta oxidation of lipids in activated BAT and other abdominal fat depots were confirmed by histology. Figures 5A, B, C shows the H & E sections from BAT, WAT and retroperitoneal fat depot of HFD fed obese rat treated with β3-adrenergic agonist and saline. The intracellular lipid droplets were reduced in BAT, treated with β3-adrenergic agonist compared to saline treated rats, indicating the utilization of lipid droplets as fuel substrates during β3-adrenergic mediated thermogenesis. We noticed the decrease in size of white adipocytes (Figure 5D) in abdominal fat depots including gonadal, mesenteric, retroperitoneal. Tiny pockets of brown adipocytes were also observed within the white adipose tissue. Reduction in adipocyte size and remodeling of white adipose tissues improved the insulin sensitivity.Conclusions
The β3-adrenergic receptor mediated stimulation significantly reduced the fat content of iBAT in HFD fed obese animals. The weight of iBAT increased with β3-adrenergic agonist treatment indicating the remodeling of WAT into BAT. Systemic effects of BAT activation also resulted in reduction of abdominal fat depots. Upregulation of CPT1 and UCP1 genes in iBAT supports the lipolysis induced beta oxidation of fat. Reduction in adipocyte size of abdominal fat depots also supports in improvement of insulin sensitivity.Acknowledgements
No acknowledgement found.References
(1). Tchernof A., Desres J, Pathophysiology of human visceral obesity: An update. Physiol Rev. 2013;93:359-404.
(2). Weyer C, et al, Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498-1506.
(3).Strosberg, D A. Structure and function of the β3-adrenergic receptor. Review of Pharmacology and Toxicology. 1997;37:421-450.
(4). Cypess A.M., et.al, Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21:33-38.
(5) Villarroya L. et al, An endocrine role for brown adipose tissue?. Am J Physiol Endocrinol Metab. 2013;305:E567-E572.
Figures