4961
A New System to Spatially Align In Vivo MRI, Ex Vivo MRI, and Whole-Mount Histopathology Slides for Integrated Prostate Cancer Research1Radiological Sciences, UCLA, Los Angeles, CA, United States, 2Bioengineering, UCLA, Los Angeles, CA, United States, 3Radiology, Cornell University, New York, NY, United States, 4Pathology, UCLA, Los Angeles, CA, United States, 5Pathology, Duke University, Durham, NC, United States, 6Urology, UCLA, Los Angeles, CA, United States
Synopsis
Multi-parametric MRI is an indispensable tool for prostate cancer (CaP) management and spatial alignment of in vivo MRI to histopathology is critical for its development. In addition, ex vivo MRI has distinct advantages for investigating ultrahigh-resolution MRI and quantitative MRI of CaP. In this work, we propose a new system for spatial alignment of in vivo MRI, ex vivo MRI, and whole-mount histopathology slides. Results from a pilot study of CaP patients demonstrate successful integration with the clinical workflow and good spatial alignment of the image sets. This new system may enable novel research of CaP biomarkers and predictive models.
Introduction
Multi-parametric MRI provides important information for the management of prostate cancer (CaP)1. The validation and development of MRI relies on comparisons with histopathology and accurate spatial alignment between the two is critical. Several alignment strategies have been proposed, including patient-specific prostate molds2,3 and ex vivo MRI of the resected prostate4–8. In addition, ex vivo MRI has distinct advantages over in vivo MRI (e.g., no patient motion) that enable ultrahigh-resolution MRI and multi-component quantitative MRI of CaP9–12. The integration of in vivo MRI, ex vivo MRI, and histopathology may enable development of new biomarkers and predictive models of CaP. However, the majority of existing strategies for spatial alignment do not fully consider the synergy between the patient-specific mold and ex vivo MRI, do not standardize the ex vivo MRI setup, or do not fully integrate into the clinical workflow. In this work, we propose a new system for spatial alignment of in vivo MRI, ex vivo MRI, and whole-mount (WM) slides that addresses these limitations. A pilot study of CaP patients is performed to evaluate workflow integration and system performance.Methods
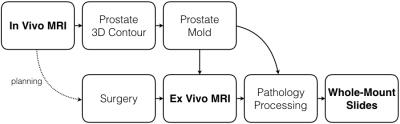
System Overview: Our clinical workflow already utilizes patient-specific molds to create WM slides3. We embedded ex vivo MRI into this workflow (Figure 1). The patient-specific mold serves as a central link and was re-designed to maintain consistent orientation and shape of the prostate throughout all steps. This study was approved by our institutional review board and biosafety committee.
In Vivo MRI Protocol: Our protocol13 included multi-planar 2D T2-weighted (T2w) MRI, 3D T2w MRI (SPACE, TE/TR=204/2230 ms, FOV 170x170x90 mm3, interpolated resolution 0.66x0.66x1.5 mm3, parallel imaging factor 2, 2 averages, 7 min), T1-weighted MRI, diffusion-weighted MRI and ADC mapping, T1 mapping, and dynamic contrast-enhanced MRI. The prostate was contoured on T2w-SPACE to design a patient-specific mold.
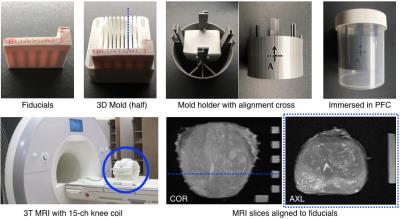
New Mold Design: The patient-specific mold (Figure 2) was 3D-printed (Replicator 2, MakerBot Industries) with slits (4.5-mm spacing) to guide sectioning. A cartridge with agar fiducial markers was inserted in the mold to aid alignment of MRI slices to the slits. The mold was inserted into a holder with an alignment cross. The mold and holder were immersed in a perfluorocarbon (PFC) solution (Fomblin, Solvay) to match the magnetic susceptibility of tissue while avoiding background MRI signal. The mold had a mesh interior for the PFC solution to permeate.
Ex Vivo MRI Protocol: The container with the prostate mold was inserted into a 15-channel knee coil on a whole-body 3T MRI scanner (Prisma, Siemens). The cross on the mold holder was aligned under the laser of the scanner for consistent positioning. The protocol included T1 mapping, 3D T2w-SPACE MRI, high-resolution T2w MRI (2D TSE, TE/TR=55/14250 ms, FOV 75x75 mm2, acquired in-plane resolution 0.29x0.29 mm2, slice thickness 1.5 mm, 3 averages, 8 min), T2 mapping, and high-resolution diffusion-weighted MRI and ADC mapping. Ex vivo T2w-TSE was used to evaluate spatial alignment with in vivo T2w-SPACE and WM slides.
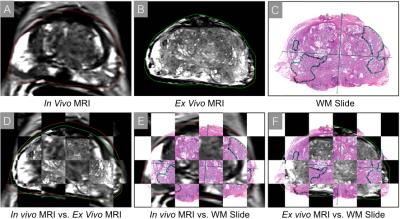
Evaluation of Spatial Alignment: A genitourinary radiologist contoured the prostate capsule and identified corresponding non-cancerous landmarks on all three image sets. The image sets were registered, starting with a rigid transformation defined by the agar fiducial positions. When registering WM slides to MRI, additional spline-based non-rigid registration was performed using control points from the prostate capsule3. Prostate contour overlap, out-of-slice error, and 3D target registration error (TRE) of landmarks were calculated.
Results
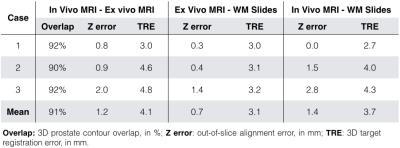
N=3 patients who underwent prostatectomy were included for pilot evaluation of our system (Table 1). We successfully completed ex vivo MRI of the fresh prostate specimen in all cases (average of 2 hours). There was no adverse impact on pathology processing. A representative case (Figure 3) shows the consistent orientation, shape, and slice position of the prostate on all image sets. Quantitative measurements (Table 2) demonstrate good spatial alignment.Discussion
Initial data shows excellent alignment between in vivo MRI and ex vivo MRI (contour overlap >90%). The slice alignment among all three image sets was ensured by the mold and out-of-slice error (average <1.5 mm) was within one MR image slice. TRE was 3-4 mm, which is consistent with previous studies7,8. However, TRE is based on subjective identification of landmarks and may have resulted in overestimation of the actual error. Further work will investigate improvements in calculating alignment error.Conclusion
We have successfully integrated the new system with our clinical workflow to achieve spatial alignment among in vivo MRI, ex vivo MRI, and WM slides. This new system may enable novel research of integrated CaP biomarkers and predictive models that combine information from multiple scales.Acknowledgements
The authors thank Dr. Isabel Dregely, Dr. Novena Rangwala, Dr. Shyam Natarajan, Dr. Leonard Marks, Dr. Sarah Dry, Clara Magyar, Voicu Ciobanu, Hayley Veal, Bryan Radosavcev, Tyler Burdick, and Zhongyi Zhang for their assistance and support. This work was supported by funds from the Integrated Diagnostics Program, Department of Radiological Sciences & Pathology, David Geffen School of Medicine at UCLA.References
1. Hegde J V, Mulkern R V, Panych LP, Fennessy FM, Fedorov A, Maier SE, Tempany CMC. Multiparametric MRI of Prostate Cancer?: An Update on State-of-the-Art Techniques and Their Performance in Detecting and Localizing Prostate Cancer. J Magn Reson Imaging 2013;37:1035–1054. doi: 10.1002/jmri.23860.
2. Trivedi H, Turkbey B, Rastinehad AR, et al. Use of Patient-specific MRI-based Prostate Mold for Validation of Multiparametric MRI in Localization of Prostate Cancer. Urology 2012;79:233–239. doi: 10.1016/j.urology.2011.10.002.
3. Priester A, Natarajan S, Khoshnoodi P, Margolis DJ, Raman SS, Reiter RE, Huang J, Grundfest W, Marks LS. MRI Underestimation of Prostate Cancer Geometry: Use of Patient-Specific Molds to Correlate Images with Whole-Mount Pathology. J. Urol. 2016:1–7. doi: 10.1016/j.juro.2016.07.084.
4. Park H, Piert MR, Khan A, Shah R, Hussain H, Siddiqui J, Chenevert TL, Meyer CR. Registration Methodology for Histological Sections and In Vivo Imaging of Human Prostate. Acad. Radiol. 2008;15:1027–1039. doi: 10.1016/j.acra.2008.01.022.
5. Gibson E, Crukley C, Gaed M, Gómez J a., Moussa M, Chin JL, Bauman GS, Fenster A, Ward AD. Registration of prostate histology images to ex vivo MR images via strand-shaped fiducials. J. Magn. Reson. Imaging 2012;36:1402–1412. doi: 10.1002/jmri.23767.
6. Orczyk C, Mikheev A, Rosenkrantz A, Melamed J, Taneja S, Rusinek H. Imaging of prostate cancer: A platform for 3D co-registration of in-vivo MRI ex-vivo MRI and pathology. Proc. SPIE 2012;8316:83162M–1. doi: 10.1117/12.911369.
7. Meyer C, Ma B, Kunju LP, Davenport M, Piert M. Challenges in accurate registration of 3-D medical imaging and histopathology in primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2013;40:72–78. doi: 10.1007/s00259-013-2382-2.
8. Reynolds HM, Williams S, Zhang A, et al. Development of a registration framework to validate MRI with histology for prostate focal therapy. Med. Phys. 2015;42:7078–7089. doi: 10.1118/1.4935343.
9. Madabhushi A, Feldman MD, Metaxas DN, Tomaszeweski J, Chute D. Automated detection of prostatic adenocarcinoma from high-resolution ex vivo MRI. IEEE Trans. Med. Imaging 2005;24:1611–25. doi: 10.1109/TMI.2005.859208.
10. Fan X, Haney CR, Agrawal G, et al. High-resolution MRI of excised human prostate specimens acquired with 9.4T in detection and identification of cancers: Validation of a technique. J. Magn. Reson. Imaging 2011;34:956–961. doi: 10.1002/jmri.22745.
11. Chatterjee A, Watson G, Myint E, Sved P, McEntee M, Bourne R. Changes in Epithelium, Stroma, and Lumen Space Correlate More Strongly with Gleason Pattern and Are Stronger Predictors of Prostate ADC Changes than Cellularity Metrics. Radiology 2015;277:751–762.
12. Hall MG, Bongers A, Sved P, Watson G, Bourne RM. Assessment of non-Gaussian diffusion with singly and doubly stretched biexponential models of diffusion-weighted MRI (DWI) signal attenuation in prostate tissue. NMR Biomed. 2015;28:486–495. doi: 10.1002/nbm.3273.
13. Tan N, Margolis DJ, Lu DY, King KG, Huang J, Reiter RE, Raman SS. Characteristics of Detected and Missed Prostate Cancer Foci on 3-T Multiparametric MRI Using an Endorectal Coil Correlated With Whole-Mount Thin-Section Histopathology. Am. J. Roentgenol. 2015;205:W87–W92. doi: 10.2214/AJR.14.13285.
Figures