4956
Prostate shapes between prostate cancer patients with and without biochemical recurrence post-treatment are different : Preliminary study1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2GE Global Research, 3Perelman School of Medicine, University of Pennsylvania, 4Diagnostic Radiology, Cleveland Clinic Foundation, Cleveland, OH, 5Diagnostic Radiology, Cleveland Clinic Foundation, Cleveland, OH, United States, 6Urology, Case Western Reserve University School of Medicine
Synopsis
In a single center IRB approved retrospective study, statistically significant differences in the shape of the prostate gland were observed between BCR+ and BCR- populations.
Introduction
Despite advancement in surgical procedures and radiation therapy, there may be treatment failure or biochemical recurrence (BCR) of prostate cancer in estimated 30-35% of the treated prostate cancer (PCa) patients within 10 years of definitive therapy1. Patients developing BCR are at higher risk of mortality due to disease progression. Hence early prediction of BCR may enable the use of more aggressive or neo-adjuvant therapies and may potentially improve patient outcome. BCR is often associated with aggressive cancers, resulting in an abnormal bulge in the prostate gland 2. In many instances extracapsular (ECap) spread which is found to be predictive of BCR is associated with an irregular bulge in the prostate, focal capsular retraction and or thickening broad capsular tumor contact3. These findings beg the question whether there are quantitative differences in the shape between the prostate capsules for patients who will (BCR+) and will not go on to develop BCR (BCR-) following definitive therapy (i.e. surgery or radiation). In this study we seek to explore this question by quantitatively analyzing and comparing the shape of prostate capsule as captured on pre-operative T2w MRI between BCR+ and BCR- patients.Materials and Methods
A single center IRB approved study included 874 patients who were referred for prostate MRI between 2008 and 2014. The inclusion criteria were as follows: availability of complete image datasets (T1w, T2w and ADC map); no treatment for PCa before MRI; presence of clinically localized PCa; availability of Gleason score; and data available for post-treatment PSA and follow-up for at least 3 years in patients without BCR. Of the 874 patients in the registry, 77 patients fit these criteria. To reduce statistical bias, the following rules were employed for cohort selection: a) equal number of patients in BCR+ and BCR- groups; b) similar Gleason scores in both groups; and c) similar tumor stages in both groups. This reduced the final number of study patients to 50. Of these 50 patients, 25 were in the BCR+ group and 25 were in the BCR- group. The prostate capsule was manually segmented on T2w images by an experienced genitourinary radiologist with more than 7 years of experience in reading prostate MRI. To perform a statistical comparison of prostate gland shapes between BCR+ and BCR- cases, all prostate MRIs were aligned to a common space. Registration to the representative template was performed in two stages using an initial affine registration4, followed by a non-rigid B-spline5 based registration. Two atlases, A+ and A-, were constructed, one each for the BCR+ and the BCR- groups respectively. Each of A+ and A- were affine registered to bring both the atlases to a common space for statistical comparison. All registered prostate capsules of both the BCR+ and BCR- groups are transformed into a signed distance function using Danielsson distance map function6. As opposed to the binary representation of a mask where each voxel within the prostate capsule is 1 and 0 outside, each voxel determines the distance of a given voxel from the capsule boundary. The function has positive values for voxels inside the prostate capsule, it decreases in value as the voxel approaches the boundary where the signed distance function is zero, and it takes negative values outside of the prostate capsule. The statistical comparisons of BCR+ and BCR- is now done using the corresponding signed distance representations of each registered prostate capsule. A non-parametric General Linear Model (GLM) based t-test8 with permutation testing and corrections for multiple comparison testing was used to find statistically significant prostate capsule shape differences between A+ and A-. The effect of ECap spread on prostate capsule shape was also analyzed.Results
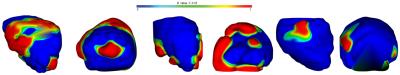
The comparison of A+ and A- atlases revealed statistically significant differences (p<0.05) in the shape of the prostate capsule. Statistically significant shape differences in the prostate capsule were observed between BCR+ without ECap spread and BCR- without ECap spread groups. Statistically significant shape differences in prostate shapes between BCR+ with ECap spread and BCR- with ECap spread groups was also observed. These results are presented in Figure 1. Between the A+ and A- atlases, statistically significant shape differences were identified on the anterior, posterior and the lateral side of the prostate. Comparing patients with ECap [AM1] spread, statistically significant differences were observed primarily towards the anterior, lateral and posterior parts of the prostate. Comparing patients without ECap spread, statistically significant shape differences were identified towards the posterior and lateral sides of the prostate. [AM1]Change extent everywhere to spreadAcknowledgements
No acknowledgement found.References
1. Boorjian, S. A. et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur. Urol. 59, 893–899 (2011).
2. Koca, O., et al. The factors predicting biochemical recurrence in patients with radical prostatectomy. Archivio Italiano di Urologia e Andrologia 87, 270–275 (2016).
3. Davis, R. et al. Accuracy of multiparametric magnetic resonance imaging for extracapsular extension for prostate cancer in community practice. Clinical Genitourinary Cancer (2016).
4. Ourselin, S., et al. Reconstructing a 3d structure from serial histological sections. Image and vision computing 19, 25–31 (2001).
5. Rueckert, D. et al. Nonrigid registration using free-form deformations: application to breast MR images. Medical Imaging, IEEE Transactions on 18, 712–721 (1999).
6. Danielsson, P.-E. Euclidean distance mapping. Computer Graphics and Image Processing 14, 227 – 248 (1980).
7. Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M. & Nichols, T. E. Permutation inference for the general linear model. Neuroimage 92, 381–397 (2014).
Figures