4930
Diffusion Kurtosis as an in vivo Imaging Marker for Characterizing Breast Carcinoma: Correlation with Cellular Proliferation1Department of Radiology, 2nd Affiliated Hospital of Shantou University Medical College, China, Shantou, People's Republic of China, 2Department of General Surgery, 2nd Affiliated Hospital of Shantou University Medical College, China, Shantou, People's Republic of China, 3Department of Radiology,1st Affiliated Hospital of Hubei Science and Technology College,Xianning Central Hospital,China, Xianning, People's Republic of China
Synopsis
This study aimed to assess the diagnostic accuracy of DKI technique in breast cancer patients, and to evaluate the potential associations between DKI-derived parameters and cellular proliferation of breast cancer. Mean kurtosis (MK) derived from DKI exhibited the maximal AUCs (0.972) and corresponding optimal sensitivity (90.2%) and specificity (95.2%) for distinguishing malignancy from benign lesions. Furthermore, positive correlation between MK and pathological prognostic factors (Ki-67 expression and histological grade) were found. Preliminary findings highlighted the potential utility of DKI as a sensitive MR sequence for imaging studies and diagnostic improvement of breast masses.
Introduction
Breast cancer (BC) is the most common cancer in women worldwide 1. Magnetic Resonance Imaging (MRI) is increasingly being used in breast cancer patients for risk stratification, treatment planning and postoperative surveillance 2. Breast MRI has primarily focused on dynamic contrast-enhanced MRI (DCE-MRI) and diffusion weighted imaging (DWI) techniques in examination. However, there were diagnositic insufficiency for the discrimination between malignancy and benign lesions, due to the substantial overlaps of the time intensity curves for DCE imaging and that of the ADC value for DWI 3. An advanced non-Gaussian diffusion-weighted model called diffusion kurtosis imaging (DKI), which involves in the calculation of kurtosis and diffusion coefficients, has the potential to increase diagnostic accuracy, as compared to conventional DWI4. This initial study aimed to assess the diagnostic accuracy of DKI technique in breast cancer patients, and to evaluate the potential associations between DKI-derived parameters and cellular proliferation of breast cancer.Methods
Fifty-five patients with suspicious breast lesions (BI-RADS IV and V lesions) were evaluated. All MRI examinations were performed on 3.0T GE scanner using a dedicated four-channel bilateral breast coil. DKI data was acquired using an echo planar imaging (EPI) diffusion sequence with following parameters: TR/TE = 6000/66ms, number of averages = 2, slice thickness = 4mm, field of view (FOV) = 32cm2, data matrix = 192×192, with six b values (0, 500, 1000, 2500, 2000, 2500 s/mm2) for each direction. DWI images (TR/TE = 4900/106ms) with two b-values (0, and 850s/mm2)5 were performed. Mean values for apparent diffusion coefficient (ADC), mean kurtosis (MK) and mean diffusivity (MD) were determined by two blinded radiologists in consensus. Receiver operating characteristics (ROC) analysis was performed to evaluate the diagnostic accuracy of DKI based on MD and MK thresholds.Tumor cellularity was measured by the percentage of Ki-67 positive tumor cell nuclei according to St Gallen International Expert Consensus6. All data were statistically analyzed using SPSS 20.0 with p<0.05.Results
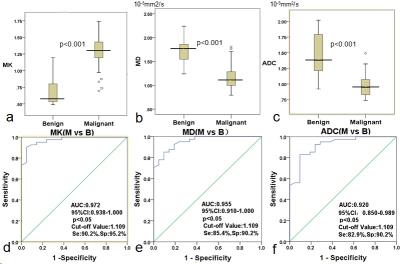
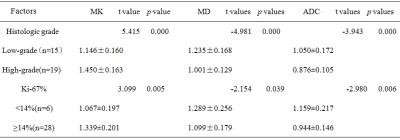
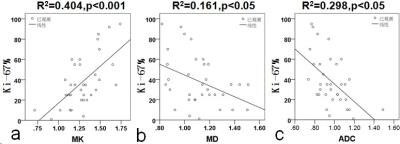
Histopathology confirmed malignancy in 61.8% (34/55) and benign in 38.2% (21/55) of lesions.As shown in Table 1, the mean ADC, MD and MK of malignant lesions were(0.958±0.163)×10-3 mm2/s,(1.106±0.185)×10-3 mm2/s and(1.311±0.218), and the mean ADC, MD and MK of benign lesions were(1.435±0.316)×10-3 mm2/s,(1.637±0.270)×10-3 mm2/s and(0.724±0.216), respectively. Significant differences were obtained between benign and malignant lesions for all parameters(ADC, t=7.87, p<0.001; MD,t=9.11, p<0.001; MK, t=-10.07, p<0.001). The area under the ROC curve (AUC) of ADC, MD and MK between malignant and benign lesions was 0.920, 0.955 and 0.972, respectively. Taken the maximum Youden`s index of ADC (1.109×10-3 mm2/s), MD (1.309×103 um2/s) and MK (1.109) as the ROC optimal cut-off point, the sensitivity of ADC, MD and MK were 82.9%, 85.4% , 90.2%, respectively, and their corresponding specificity were 90.2%, 90.2% and 95.2%, for the diagnosis of malignant lesions (Figure 1). Figure 2 and Table2 showed the potential associations between DKI-derived parameters (ADC, MD, MK) and histological prognosis factors of breast cancer. MK value was positively correlated with Ki-67 expression (MK: r =0.635, [95% CI: 0.387, 0.792], p<0.05) and tumor histologic grade (MK:r=0.730,[0.563,0.893]. The values of MD and ADC were negatively correlated with Ki-67 expression (MD: r =-0.401, [95% CI: -0.122, -0.633], p<0.05; ADC: r =-0.544, [95% CI: -0.305, -0.718], p<0.05) and histologic grade (MD: r =-0.610, [95% CI: -0.400, -0.760], p<0.05; ADC: r =-0.556, [95% CI: -0.296, -0.736], p<0.05).Disscussion
Our initial study demonstrated that the mean value of MK derived from DKI was higher in breast malignancy than benign lesion, and showed a reciprocal behavior to MD and ADC metrics. Kurtosis was believed to be generally proportional to the heterogeneity and complexity of the microstructure7, and malignant lesion tended to have greater structural complexity and heterogeneity compared with benign lesion. High-grade tumors demonstrated higher MK values compared to low-grade tumors. Possible reason might be due to the fact that high-grade tumors are characterized by the absence of tubule and gland formation, marked degree of nuclear pleomorphism, and frequent mitotic counts, demonstrating the increasing microstructural complexity. In addition, higher cellularity, more nuclear atypia and higher pleomorphism of breast carcinoma might account for the positive association between MK value and pathological prognostic factors (grade and Ki-67 expression).Conclusion
MK generated from DKI enables differentiation of breast lesions with a high sensitivity and specificity, highlighting the potential utility of DKI as a sensitive MR sequence for imaging studies and diagnostic improvement of breast masses.Moreover,MK might offered greater potential to noninvasively predict the cellular proliferation of breast cancer compared with conventional DWI.Acknowledgements
National Natural Science Foundation of China (81471729, 81101102), the Science and Technology Planning Project of Guangdong Province ( 2016A020216025), the Research Award Fund for Outstanding Young Teachers in Higher Education Institutions, Guangdong Province (YQ2015245), and the National Natural Science Foundation of Guangdong Province (S2011010004973).References
1. Fan L, Strasser-Weippl K, Li J-J et al: Breast cancer in China. The Lancet Oncology 2014, 15(7):e279-e289.
2. Sun K, Chen X, Chai W et al: Breast Cancer: Diffusion Kurtosis MR Imaging-Diagnostic Accuracy and Correlation with Clinical-Pathologic Factors. Radiology 2015, 277(1):46-55.
3. Partridge SC, McDonald ES: Diffusion weighted magnetic resonance imaging of the breast: protocol optimization, interpretation, and clinical applications. Magnetic resonance imaging clinics of North America 2013, 21(3):601-624.
4. Jensen JH, Helpern JA, Ramani A et al: Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magnetic resonance in medicine 2005, 53(6):1432-1440.
5. Bogner W, Gruber S, Pinker K et al: Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology 2009, 253(2):341-351.
6. Goldhirsch A, Winer EP, Coates AS et al: Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2013, 24(9):2206-2223.
7. Jiang R, Jiang J, Zhao L et al: Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget 2015, 6(39):42380-42393.
Figures