4928
3T full breast diffusion imaging at sub-millimeter resolution with high immunity to artifacts1chemical Physics, Weizmann Institute of Science, Rehovot, Israel, 2Radiology, Sheba-Medical-Center, 3Life Sciences Core Facilities, Weizmann Institute of Science, Rehovot, Israel, 4Sackler School of Medicine,Tel Aviv University
Synopsis
SPatio-temporal ENcoding (SPEN) MRI has been recenlty employed to quantify apparent diffusion coefficients in breast and in other challenging organs, thanks to its high immunity to B0-inhomogeneities and to chemical shift heterogeneities. In this study a new SPEN protocol is proposed combing multi-band pulses providing full coverage of both breasts with improved signal-noise-ratio, and multi-shot interleaved acquisitions achieving sub-millimeter spatial resolution. This provides a representation of the anatomical features that is similar to TSE, plus diffusion information containing insight into a lesion’s nature. Validations and examples are shown at 3T, including healthy female volunteers and patients with breast malignancies.
Motivation
Diffusion weighted imaging (DWI) is a valuable tool in breast cancer diagnosis, with the potential to provide insight without contrast [1-3]. Breast DWI requires robust single-shot MR methods, to ensure reliable Apparent-Diffusion-Coefficient (ADC) mapping in this organ’s challenging environment. Mainstream single-shot methods like spin-echo (SE) EPI, however, are prone to display image artifacts when applied to human breast –including geometric distortions, ghosting and imperfect fat-suppression. SPatio-temporal ENcoding (SPEN) is a single-shot technique that has proven as a highly robust alternative to SE-EPI, in terms of overcoming B0-inhomogeneities and of operating in heterogeneous chemical environments [4]. Preliminary studies incorporating SPEN into breast DW schemes have shown this method’s potential to overcoming SE-EPI’s characteristic limitations [4-7]. However, the clinical utility of this tool has so far been impaired by limited breast tissue coverage [8]. Herein, we present SPEN improvements capable of delivering full-coverage diffusion breast images with sub-millimeter in-plane resolution, in ca. 6 min acquisitions. SPEN-derived DW and diffusion tensor (DT) maps were compared vis-à-vis SE-EPI results among healthy female volunteers and in cancer patients; in both cases, SPEN’s advantages were clearly evidenced.Methods
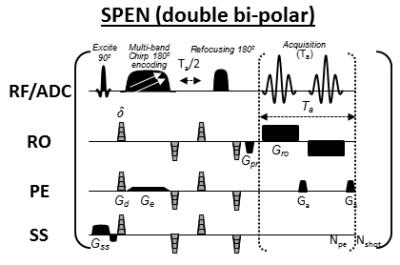
This IRB-approved study included 5 healthy volunteers and 13 patients with suspected malignancies. Axial images were acquired at 3T using a Siemens Trio scanner and a 4-channels breast coil. The clinical MRI protocol included T2-weighted turbo-spin-echo (T2w-TSE) and subtracted dynamic-contrast-enhanced (sub-DCE) T1-weighted sequences; it also included diffusion studies comparing twice-refocused SE-EPI [9], against the novel double-bipolar SPEN sequence introduced in Figure 1. This incorporates a number of improvements vis-à-vis previous implementations [8], namely: a) multi band pulses covering both breasts in a single shot, resulting in shorter scan times with improved signal-noise-ratio; b) multi-shot SPEN interleaving along the low-bandwidth axis in a reference-less, motion-immune fashion [10]; and c) partial Fourier transform along the readout. The first of these features enables low-SAR full-organ coverage, while the latter two yield sub-millimeter resolution. Diffusion measurement parameters were: b-value = 500 s/mm2 applied in 3 orthogonal and in 12 directions. Spatial resolutions were 2.0×2.0×2.5mm with single-shot DWI SPEN and SE-EPI, and 0.66×0.87×1mm when SPEN was set to perform “zoomed” single breast DTI mapping. SPEN ADC maps were obtained after suitably correcting the b-values to account for all the non-diffusion-related, imaging gradients [11].Results and Discussion
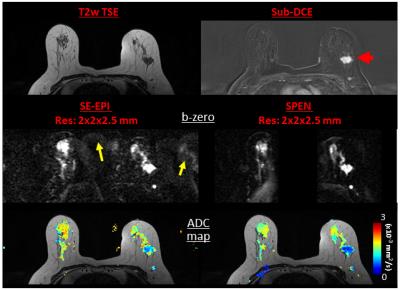
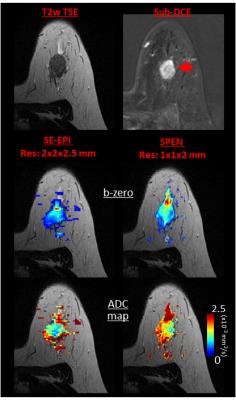
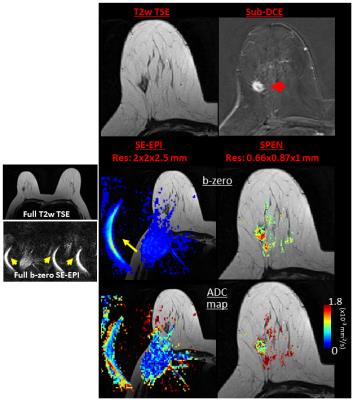
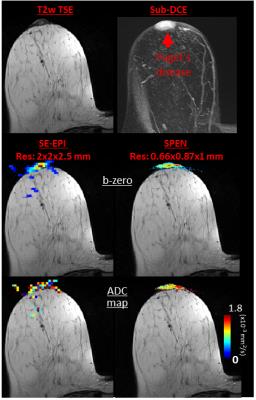
Comparisons between the DW images confirmed that many artifacts arising in SE-EPI are systematically diminished in SPEN. An example of this is given in Figure 2, with a slice of a patient exhibiting an Invasive Ductal Carcinoma (IDC). The b-zero images (middle panel) clearly demonstrate that unlike SE-EPI, SPEN MRI is free from axial artifacts and from ghosting problems surrounding and overlapping with the breast’s regions of interest, thanks to its use of a relatively high acquisition bandwidth (1.5kHz over 12cm). Notice as well SPEN’s ability to separate fat from fibroglandular tissues, and its better representation of the anatomical features when compared against a TSE reference. For both SE-EPI and SPEN, ADC maps obtained after masking background signal and non-fibro-glandular tissues (Figure 2, lower panel) clearly illustrate the lesion with its typically slower diffusion values. Figure 3, showing slices from another breast patient (with IDC), compares a higher resolution 1.0×1.0×2.0mm image arising from SPEN vs 2.0×2.0×2.5mm SE-EPI data. Based on the magnitude b-zero images, one can appreciate how SPEN preserves the original shape of the lesion. As a result, SPEN’s ADC values (lower panel) become more reliable and faithful to the anatomy than SE-EPI’s. Figure 4, a slice of a patient exhibiting Invasive Lobular Carcinoma (ILC), compares a sub-millimeter high resolution image (0.66×0.87×1.0mm) afforded by 4-shot interleaved SPEN vs a 2.0×2.0×2.5mm by SE-EPI. While SE-EPI fails to show the lesion due to its poor b-zero image (see additional full breast images), SPEN’s higher resolution succeeds to separate and characterize the lesion. Figure 5 shows a sub-millimeter SPEN DTI example of a patient with Paget's disease of the breast nipple. SE-EPI’s low resolution in combination with strong tissue/air inhomogeneities arising near the nipple, prevent it from tackling this case. By contrast, SPEN’s robustness succeeds to provide the required diffusion information.Conclusions
Extensive results demonstrate SPEN’s new capabilities to deliver reliable ADC maps at sub-mm resolution using conventional imaging acquisition hardware, while showing significantly higher image qualities and reduction of artifacts than SE-EPI. Reductions in ADCs due to the presence of malignancies can then be rapidly and non-invasively diagnosed by single-shot or interleaved SPEN MRI.Acknowledgements
This work was funded by the Israel Science Foundation grant 795/13, by ERC-2014-PoC grant # 633888, by the Minerva Foundation grant 712277, by the Kimmel Institute of Magnetic Resonance (Weizmann Institute), and by the generosity of the Perlman Family Foundation. We are also grateful to Dr. Sagit Shushan (Wolfson Medical Center), and the Weizmann MRI technical team (Fanny Attar and Nachum Stern) for assistance in the human scans.References
[1] Sardanelli, Francesco, et al. "Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group." European journal of cancer 46.8 (2010): 1296-1316.
[2] EI Khouli, Riham H., et al. "Diffusion-weighted Imaging Improves the Diagnostic Accuracy of Conventional 3.0-T Breast MR Imaging 1." Radiology 256.1 (2010): 64-73.
[3] Guo, Yong, et al. "Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging." Journal of magnetic resonance imaging 16.2 (2002): 172-178.
[4] Schmidt, Rita, and Lucio Frydman. "In vivo 3D spatial/1D spectral imaging by spatiotemporal encoding: A new single-shot experimental and processing approach." Magnetic resonance in medicine 70.2 (2013): 382-391.
[5] Schmidt, Rita, et al. "Super-resolved parallel MRI by spatiotemporal encoding." Magnetic resonance imaging 32.1 (2014): 60-70.
[6] Solomon, Eddy, et al. "Robust diffusion tensor imaging by spatiotemporal encoding: Principles and in vivo demonstrations." Magnetic resonance in medicine (2016).
[7] Solomon, Eddy, et al. "Removing silicone artifacts in diffusion-weighted breast MRI by means of shift-resolved spatiotemporally encoding." Magnetic resonance in medicine (2015).
[8] Solomon, Eddy, et al. "Overcoming limitations in diffusion-weighted MRI of breast by spatio-temporal encoding." Magnetic resonance in medicine 73.6 (2015): 2163-2173.
[9] Reese, T. G., et al. "Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo." Magnetic Resonance in Medicine 49.1 (2003): 177-182.
[10] Schmidt, Rita, Amir Seginer, and Lucio Frydman. "Interleaved multishot imaging by spatiotemporal encoding: A fast, self-referenced method for high-definition diffusion and functional MRI." Magnetic resonance in medicine(2015).
[11] Solomon, Eddy, Noam Shemesh, and Lucio Frydman. "Diffusion weighted MRI by spatiotemporal encoding: analytical description and in vivo validations." Journal of Magnetic Resonance 232 (2013): 76-86.
Figures