4924
Intravoxel motion diffusion-weighted imaging and dynamic contrast-enhanced MRI of breast: comparison of perfusion-related parameters1Beijing Hospital, Beijing, People's Republic of China, 2Beijing Hospital, 3GE Healthcare, 4Chaoyang Hospital
Synopsis
IVIM is a research and clinical focus in recent years. Whether its perfusion-related parameters are correlated with those obtained from DCE is still under debate. So the purpose is to investigate their correlation by performing both IVIM and DCE on 31 malignant and 35 benign lesions from 59 patients. Their diagnostic performance and correlation were investigated. No strong correlation was found between them, although their diagnostic performance is similar in terms of perfusion parameters. So IVIM is useful in lesion differentiation and potentially comparable with DCE-derived perfusion-related parameters. IVIM-derived perfusion-related parameters are probably a new entity of microcirculation parameters.
ABSTRACT
Purpose: Dynamic contrast-enhanced (DCE) sequence, which can provide both perfusion-related and morphological information of lesion, has become the routine for breast MRI. But how to balance between temporal and spatial resolution is always a dilemma. In addition, DCE-breast MRI cannot provide perfusion information without intravenous contrast media. Intravoxel incoherent motion (IVIM) imaging is a non-contrast-enhanced diffusion-weighted imaging (DWI) technique, initially reported by Le Bihan et al 1. It has not been clinically applied until MR hardware is dramatically improved in recent years. Several of the studies 2-4 investigated correlation of perfusion-related parameters from IVIM with those from contrast enhanced MRI, showing significant correlation of D* and f values with relative cerebral blood volume in brain and with vascular plasma volume and transfer constant in prostate. However, only few studies 5-9 investigated the application of IVIM in breast. This is important for further exploring the potential of IVIM as an alternative to DCE for individuals who cannot tolerate contrast media. So the purpose of the study is to compare the diagnostic performance of perfusion-related parameters obtained with IVIM and model-based and model-free perfusion-related parameters from DCE, and to investigate their correlation.
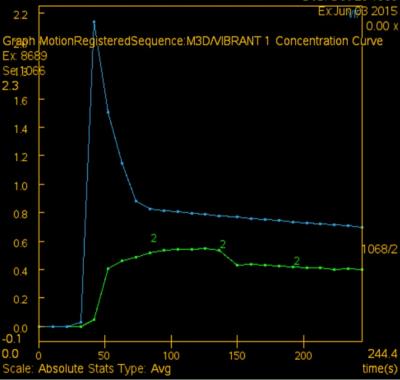
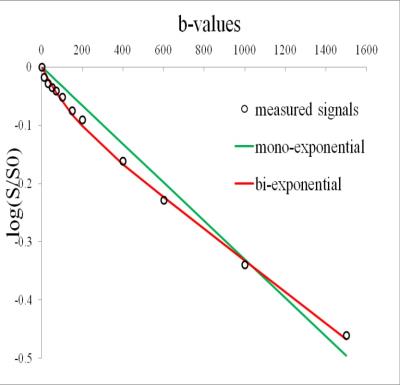
Material and methods: The prospective study protocol was approved by our institutional review board. Inclusion criteria for the MR protocol were as follows: 1, patients with breast lesions who are indicated for further MR imaging; 2, no general contraindications to MR imaging and contrast media; 3, no breast biopsy, surgery or chemotherapy has been performed before MRI. From March 2015 to April 2016, 66 (31 malignant and 35 benign) pathology-proven breast lesions from 59 patients were finally included. A hybrid DCE sequence 10, combining high-temporal and high spatial resolution, and IVIM using 12 b values (0, 10, 30, 50, 70, 100, 150, 200, 400, 600, 1000, 1500 s/mm2) were performed on a 3T MR unit. Parameters from IVIM included: apparent diffusion coefficient (ADC), true diffusion coefficient (D), perfusion fraction (f) and pseudo-diffusion coefficient (D*). The following Tofts’ model-based 11,12 and model-free parameters were calculated from DCE: forward volume transfer constant (Ktrans), extravascular extracellular space volume per unit volume of tissue (Ve), reverse volume transfer constant (Kep), time intensity curve (TIC), positive enhancement integral (PEI), contrast enhancement rate (CER), initial area under the concentration curve (IAUGC), maximum slope of increase (MaxSlope). Lesion discrimination based on these parameters was assessed by receiver operating curve (ROC) analysis. Correlations between IVIM and DCE parameters were analyzed by Spearman’s test.
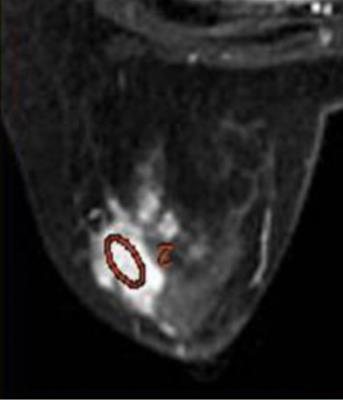
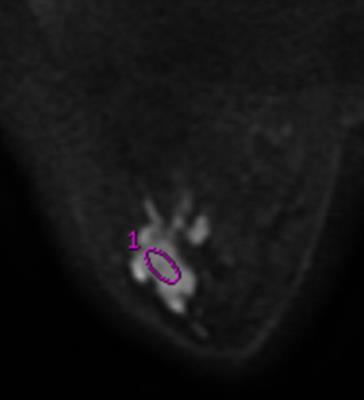
Results: TIC, IAUGC, MaxSlope, Ktrans, Kep, ADC, D, D*and f in malignancy were significantly different from those of benign lesions (p=0.000, 0.000, 0.001, 0.000, 0.000, 0.000, 0.000, 0.045, 0.000, respectively). There was no significant difference, as to PEI, CER, Ve. In ROC analysis, AUCs of TIC, IAUGC, MaxSlope, Ktrans, Kep, ADC, D, D*and f were 0.788, 0.788, 0.736, 0.860, 0.782, 0.876, 0.909, 0.644 and 0.815, respectively. No strong correlation was found between f or D* and any DCE-derived parameters. A representative case is shown in Fig 1-5
Discussion: IVIM MR imaging could assess both tissue perfusion and diffusion simultaneously. D and f are good discriminator in terms of differentiating benign and malignant breast lesions, concordant with literature. In ROC analysis, AUC of f is similar to those of the perfusion-related parameters obtained from DCE, indicating comparable diagnostic performance and its potential ability as a surrogate to DCE in terms of reflecting lesions’ perfusion property. D* in the present study demonstrated border-line significant difference between the malignant and benign, and poor diagnostic performance, probably because of its relatively poor reproducibility and variable values. No strong correlation between f or D* and any DCE-derived parameters was presumably due to several reasons. First, the perfusion-related parameters of IVIM and DCE are based on different theoretical background. Henkelman13 suggested that IVIM does not measure tissue perfusion but rather the flow in the direction of the velocity-encoding gradient, whereas classical perfusion measures blood delivery. Second, the sequence of DCE in this study was specially designed, which may decrease the accuracy of perfusion-related parameters of DCE. There are several limitations, such as hybrid DCE sequence, small study cohort.
Conclusion: IVIM not only provides valuable diffusion information, f value is also useful in lesion differentiation and potentially comparable with DCE-derived perfusion-related parameters. However, D* shows low diagnostic performance. IVIM-derived perfusion-related parameters are probably a new entity of microcirculation parameters, different from those obtained from DCE.
Keywords: intravoxel incoherent motion, diffusion-weighted imaging, breast, dynamic contrast-enhanced MRI
Acknowledgements
Thank MR Director M. Chen, technician Ch. Zhang, Pathologist Zh. Wang, Dr. M. Zhang, and W.Xiao for their great help.References
1 Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168(2): 497–505.
2 Federau C, Maeder P, O’Brien K, et al. Quantitative measurement of brain perfusion with intravoxel incoherent motion MR imaging. Radiology 2012; 265(3): 874–81.
3 Bisdas S, Koh TS, Roder C, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of gliomas: feasibility of the method and initial results. Neuroradiology 2013; 55(10): 1189–96.
4 Pang Y, Turkbey B, Bernardo M, et al. Intravoxel incoherent motion MR imaging for prostate cancer: an evaluation of perfusion fraction and diffusion coefficient derived from different b-value combinations. Magn Reson Med 2013; 69(2): 553–62.
5 Suo S, Lin N, Wang H, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of breast cancer at 3.0 tesla: Comparison of different curve-fitting methods. J Magn Reson Imaging 2015; 42(2): 362-70.
6 Cho GY, Moy L, Zhang JL, et al. Comparison of fitting methods and b-value sampling strategies for intravoxel incoherent motion in breast cancer. Magn Reson Med 2015; 74(4):1077-85.
7 Lima M, Yano K, Kataoka M, et al. Quantitative non-Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Invest Radiol 2015; 50(4): 205-11.
8 Bokacheva L, Kaplan JB, Giri DD, et al. Intravoxel incoherent motion diffusion-weighted MRI at 3.0 T differentiates malignant breast lesions from benign lesions and breast parenchyma. J Magn Reson Imaging 2014; 40(4): 813-23.
9 Liu C, Liang C, Liu Z, et al. Intravoxel incoherent motion (IVIM) in evaluation of breast lesions: comparison with conventional DWI. Eur J Radiol 2013; 82(12): e782-9.
10 El Khouli RH, Macura KJ, Kamel IR, et al. 3T dynamic contrast- enhanced MRI of the breast: pharmacokinetic parameters versus conventional kinetic curve analysis. AJR 2011; 197(6):1498-505.
11 Tofts PS, Berkowitz B, Schnall MD. Quantitative analysis of dynamic Gd-DTPA enhancement in breast tumors using a permeability model. Magn Reson Med 1995; 33(4):564–8.
12 Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 1999; 10(3):223–32.
13 Henkelman RM. Does IVIM measure classical perfusion? Magn Reson Med 1990; 16(3): 470–5
Figures